From Bioblast
- high-resolution terminology - matching measurements at high-resolution
MitoPedia: Respiratory control ratios
The MitoPedia terminology is developed continuously in the spirit of Gentle Science.
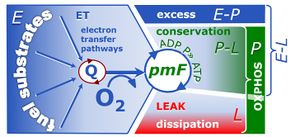
- The notion of respiratory control ratios has advanced far beyond the classical respiratory acceptor control ratio RCR, aligning the corresponding coupling control efficiencies closer with the general concept of efficiency and linking pathway control ratios with the definition of additivity (Gnaiger 2020).
MitoPedia terms: Respiratory control ratios
| Term | Abbreviation | Description |
|---|---|---|
| Biochemical coupling efficiency | jE-L | |
| CI control ratio | N/NS; CI/CI&II | See N/NS pathway control ratio |
| CII control ratio | S/NS; CII/CI&II | See S/NS pathway control ratio |
| Coupling-control efficiency | Coupling-control efficiencies are flux control efficiencies jZ-Y at a constant ET-pathway competent state. | |
| Coupling-control ratio | CCR | Coupling-control ratios CCR are flux control ratios FCR at a constant mitochondrial pathway-control state. In mitochondrial preparations, there are three well-defined coupling states of respiration: LEAK respiration, OXPHOS, and Electron-transfer-pathway state (ET state). In these states, the corresponding respirtory rates are symbolized as L, P, and E. In living cells, the OXPHOS state cannot be induced, but in the ROUTINE state the respiration rate is R. A reference rate Z is defined by taking Z as the maximum flux, i.e. flux E in the ET-state, such that the lower and upper limits of the CCR are defined as 0.0 and 1.0. Then there are two mitochondrial CCR, L/E and P/E, and two CCR for living cells, L/E and R/E. |
| Cytochrome c control efficiency | jcyt c | The cytochrome c control efficiency expresses the control of respiration by externally added cytochrome c, c, as a fractional change of flux from substrate state CHNO to CHNOc. These fluxes are corrected for Rox and may be measured in the OXPHOS state or ET state, but not in the LEAK state. In this flux control efficiency, CHNOc is the reference state with stimulated flux; CHNO is the background state with CHNO substrates, upon which c is added: jcyt c = (JCHNOc-JCHNO)/JCHNOc. |
| E-L coupling efficiency | jE-L | |
| E-P control efficiency | jE-P | |
| E-R control efficiency | jE-R | |
| Ergodynamic efficiency | ε | The ergodynamic efficiency, ε (compare thermodynamic efficiency), is a power ratio between the output power and the (negative) input power of an energetically coupled process. Since power [W] is the product of a flow and the conjugated thermodynamic force, the ergodynamic efficiency is the product of an output/input flow ratio and the corresponding force ratio. The efficiency is 0.0 in a fully uncoupled system (zero output flow) or at level flow (zero output force). The maximum efficiency of 1.0 can be reached only in a fully (mechanistically) coupled system at the limit of zero flow at ergodynamic equilibrium. The ergodynamic efficiency of coupling between ATP production (DT phosphorylation) and oxygen consumption is the flux ratio of DT phosphorylation flux and oxygen flux (P»/O2 ratio) multiplied by the corresponding force ratio. Compare with the OXPHOS-coupling efficiency. |
| Flux control efficiency | jZ-Y | Flux control efficiencies express the control of respiration by a metabolic control variable, X, as a fractional change of flux from YX to ZX, normalized for ZX. ZX is the reference state with high (stimulated or un-inhibited) flux; YX is the background state at low flux, upon which X acts.
Complementary to the concept of flux control ratios and analogous to elasticities of metabolic control analysis, the flux control efficiency of X upon background YX is expressed as the change of flux from YX to ZX normalized for the reference state ZX. » MiPNet article |
| Flux control ratio | FCR | Flux control ratios FCRs are ratios of oxygen flux in different respiratory control states, normalized for maximum flux in a common reference state, to obtain theoretical lower and upper limits of 0.0 and 1.0 (0 % and 100 %). For a given protocol or set of respiratory protocols, flux control ratios provide a fingerprint of coupling and substrate control independent of (1) mt-content in cells or tissues, (2) purification in preparations of isolated mitochondria, and (3) assay conditions for determination of tissue mass or mt-markers external to a respiratory protocol (CS, protein, stereology, etc.). FCR obtained from a single respirometric incubation with sequential titrations (sequential protocol; SUIT protocol) provide an internal normalization, expressing respiratory control independent of mitochondrial content and thus independent of a marker for mitochondrial amount. FCR obtained from separate (parallel) protocols depend on equal distribution of subsamples obtained from a homogenous mt-preparation or determination of a common mitochondrial marker. |
| Hyphenation | Hyphenation is used to connect two words (compound words) or two parts of a word to clarify the meaning of a sentence. The same two words may be hyphenated or not depending on context. Hyphenation may present a problem when searching for a term such as 'Steady state'. It is helpful to write 'steady-state measurement', to clarify that the measurement is performed at steady state, rather than implying that a state measurement is steady. But this does not imply that hyphenation is applied to the 'measurement performed at steady state'. Thus, the key word is 'steady state'. Compound adjectives should be hyphenated (steady-state measurement), but if the compound adjective follows the term (measurement at steady state), hyphenation does not add any information and should be avoided. Find more examples and guidelines in the grammarly blog on Hyphen and in apastyle.apa.org. | |
| L/E coupling-control ratio | L/E | |
| L/P coupling-control ratio | L/P | |
| L/R coupling-control ratio | L/R | |
| Metabolic control variable | X | A metabolic control variable X causes the transition between a background state Y (background rate YX) and a reference state Z (reference rate ZX). X may be a stimulator or activator of flux, inducing the step change from background to reference steady state (Y to Z). Alternatively, X may be an inhibitor of flux, absent in the reference state but present in the background state (step change from Z to Y). |
| N/NS pathway control ratio | N/NS | The N/NS pathway control ratio is obtained when succinate is added to N-linked respiration in a defined coupling state. N and NS are abbreviations for respiration in the N-pathway control state (with pyruvate, glutamate, malate, or other ETS competent N-linked substrate combinations) and the NS-pathway control state (N in combination with succinate). NS indicates respiration with a cocktail of substrates supporting the N- and S-pathways. |
| N/S pathway control ratio | N/S | The N/S pathway control ratio is obtained from SUIT protocols when the N-pathway flux and S-pathway flux are measured in the same coupling control state. The N/S pathway control ratio may be larger or smaller than 1.0, depending on the mitochondrial source and various mitochondrial injuries. The S-pathway control state may be selected preferentially as reference state, if mitochondria are studied with respect to N-pathway injuries. |
| NS-N pathway control efficiency | jNS-N; jCI&II-CI | The NS-N pathway control efficiency, jNS-N = 1-N/NS, expresses the fractional change of flux when succinate is added to the N-pathway control state in a defined coupling-control state. |
| NS-S pathway control efficiency | jNS-S | The NS-S pathway control efficiency expresses the relative stimulation of succinate supported respiration (S) by NADH-linked substrates (N), with the S-pathway control state as the background state and the NS-pathway control state as the reference state. In typical SUIT protocols with type N and S substrates, flux in the NS-pathway control state NS is inhibited by rotenone to measure flux in the S-pathway control state, S(Rot) or S. Then the NS-S pathway control efficiency in the ET-coupling state is
j(NS-S)E = (NSE-SE)/NSEThe NS-S pathway control efficiency expresses the fractional change of flux in a defined coupling-control state when inhibition by rotenone is removed from flux under S-pathway control in the presence of a type N substrate combination. Experimentally rotenone Rot is added to the NS-state. The reversed protocol, adding N-substrates to a S-pathway control background does not provide a valid estimation of S-respiration with succinate in the absence of Rot, since oxaloacetate accumulates as a potent inhibitor of succinate dehydrogenase CII. |
| Net P/E control ratio | (P-L)/E | |
| Net R/E control ratio | (R-L)/E | |
| Normalization of rate | Normalization of rate (respiratory rate, rate of hydrogen peroxide production, growth rate) is required to report experimental data. Normalization of rates leads to a diversity of formats. Normalization is guided by physicochemical principles, methodological considerations, and conceptual strategies. The challenges of measuring respiratory rate are matched by those of normalization. Normalization of rates for: (1) the number of objects (cells, organisms); (2) the volume or mass of the experimental sample; and (3) the concentration of mitochondrial markers in the instrumental chamber are sample-specific normalizations, which are distinguished from system-specific normalization for the volume of the instrumental chamber (the measuring system). Metabolic flow, I, per countable object increases as the size of the object is increased. This confounding factor is eliminated by expressing rate as sample-mass specific or sample-volume specific flux, J. Flow is an extensive quantity, whereas flux is a specific quantity. If the aim is to find differences in mitochondrial function independent of mitochondrial density, then normalization to a mitochondrial marker is imperative. Flux control ratios and flux control efficiencies are based on internal normalization for rate in a reference state, are independent of externally measured markers and, therefore, are statistically robust. | |
| P-L control efficiency | jP-L | |
| P/E control ratio | P/E | |
| Pathway control efficiency | jZ-Y | Pathway control efficiencies are flux control efficiencies, expressing the relative change of flux in response to a transition between two electron-transfer-pathway states due to a change of (1) substrate availability or (2) inhibition of enzyme steps in the pathway, in a defined coupling-control state. |
| Pathway control ratio | FCR | Substrate control ratios are flux control ratios FCR, at a constant mitochondrial coupling-control state. Whereas there are only three well-defined coupling-control states of mitochondrial respiration, L, P, E (LEAK respiration, OXPHOS, Electron transfer pathway), numerous Electron-transfer-pathway states are possible. Careful selection of the reference state, Jref, is required, for which some guidelines may be provided without the possibility to formulate general rules. FCR are best defined by taking Jref as the maximum flux (e.g. NSE), such that flux in various other respiratory states, Ji, is smaller or equal to Jref. However, this is not generally possible with FCR. For instance, the N/S pathway control ratio (at constant coupling-control state) may be larger or smaller than 1.0, depending on the mitochondrial source and various mitochondrial injuries. The S-pathway control state may be selected preferentially as Jref, if mitochondria with variable N-linked injuries are studied. In contrast, the reference state, Z, is strictly defined for flux control efficiency. |
| R-L control efficiency | jR-L | |
| R/E control ratio | R/E | |
| Respiratory acceptor control ratio | RCR | The respiratory acceptor control ratio (RCR) is defined as State 3/State 4 [1]. If State 3 is measured at saturating [ADP], RCR is the inverse of the OXPHOS control ratio, L/P (when State 3 is equivalent to the OXPHOS state, P). RCR is directly but non-linearly related to the P-L control efficiency, jP-L = 1-L/P, with boundaries from 0.0 to 1.0. In contrast, RCR ranges from 1.0 to infinity, which needs to be considered when performing statistical analyses. In living cells, the term RCR has been used for the ratio State 3u/State 4o, i.e. for the inverse L/E ratio [2,3]. Then for conceptual and statistical reasons, RCR should be replaced by the E-L coupling efficiency, 1-L/E [4]. |
| S/NS pathway control ratio | S/NS | The S/NS pathway control ratio is obtained when rotenone (Rot) is added to the NS-pathway control state in a defined coupling control state. The reversed protocol, adding N-type substrates to a S-pathway control state as the background state does not provide a valid estimation of S-linked respiration with succinate in the absence of Rot, since oxaloacetate accumulates as a potent inhibitor of succinate dehydrogenase (CII). |
| Substrate control efficiency | See Pathway control efficiency | |
| Substrate control ratio | See Pathway control ratio | |
| Uncoupling-control ratio | UCR | The uncoupling-control ratio UCR is the ratio of ET-pathway/ROUTINE-respiration (E/R) in living cells, evaluated by careful uncoupler titrations (Steinlechner et al 1996). Compare ROUTINE-control ratio (R/E) (Gnaiger 2008). |
References
| Bioblast link | Reference | Year |
|---|---|---|
| Chance 1955 J Biol Chem-III | Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation: III. The steady state. J Biol Chem 217:409-27. | 1955 |
| Chance 1955 J Biol Chem-I | Chance B, Williams GR (1955) Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 217:383-93. | 1955 |
| Chance 1956 Adv Enzymol Relat Subj Biochem | Chance B, Williams GR (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17:65-134. | 1956 |
| Gnaiger 2009 Int J Biochem Cell Biol | Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. https://doi.org/10.1016/j.biocel.2009.03.013 | 2009 |
| Gnaiger 2020 BEC MitoPathways | Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | 2020 |
| BEC 2020.1 doi10.26124bec2020-0001.v1 | Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1 | 2020 |
- See also