Gnaiger 2008 POS
| Gnaiger E (2008) Polarographic oxygen sensors, the oxygraph and high-resolution respirometry to assess mitochondrial function. In: Mitochondrial dysfunction in drug-induced toxicity (Dykens JA, Will Y, eds) John Wiley & Sons, Inc, Hoboken, NJ:327-52. |
Gnaiger Erich (2008) John Wiley
Abstract:
Several features distinguish high-resolution respirometry from traditional oxygraphs, combined in the new Oroboros O2k (Oroboros Instruments, Innsbruck, Austria). The specifications are unique: the limit of detection of respiratory flux is 1 pmol∙s-1∙cm-3 (0.001 µM∙s-1) and the limit of detection of oxygen concentration extends to 0.005 µM O2. For the non-specialist, the O2k provides robustness and reliability of instrumental performance. With small amounts of sample and correspondingly low respiratory flux per volume, the oxygen capacity of the system is exhausted slowly, allowing sufficient time to evaluate the stability of respiratory activity in each metabolic state and to permit complex titration regimes in living cells or in permeabilized cells and tissues. To increase throughput in research with cell cultures and in the pharmacological arena, user-friendly features make it possible to apply several instruments in parallel, each O2k with two independent chambers.
• O2k-Network Lab: AT Innsbruck Gnaiger E, AT Innsbruck Oroboros
Figure legend
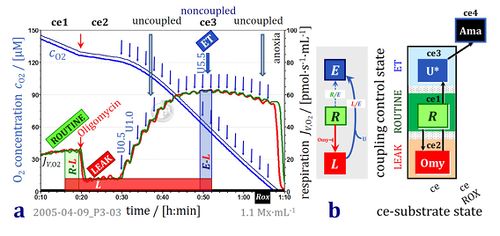
- Oxygen concentration and oxygen flux in living cells with coupling control protocol. High-resolution respirometry (Oroboros O2k with TIP2k) with parental hematopoietic 32D cells at 1.1·106 cells/cm3 suspended in culture medium RPMI at 37 °C. Replicate measurements in the two O2k-chambers (2 cm3). Superimposed plots of oxygen concentration [O2] and volume-specific oxygen flux, JV,O2, calculated as the negative time derivative of oxygen concentration. ROUTINE respiration (R) is followed by inhibition of ATP synthase (manual titration of oligomycin, 2 μg·ml-1) to induce nonphosphorylating LEAK respiration (L). Automatic titration of uncoupler (10 mM FCCP in the TIP2k) in steps of 0.1 μl corresponding to a step increase in the final concentration of 0.5 μM FCCP at intervals of 120 s. Maximum noncoupled flux (capacity of the Electron transfer-pathway, ET-capacity; state E) is reached at 5.5 μM FCCP. The L/E ratio is 0.10. Respiration is inhibited at higher [FCCP], unrelated to sample dilution (<1 %). O2k-experiment 2005-04-09 P3-03, carried out by participants of an O2k-Workshop (Gnaiger 2020 BEC MitoPathways).
Coupling control protocol
- CCP: 1ce;2ceOmy;3ceU-
- 1ce: addition of living cells (ce); measurement of ROUTINE respiration.
- 2ceOmy: Oligomycin is permeable through the plasma membrane of living cells (ce); measurement of LEAK respiration.
- 3ceU: Uncoupler titration, uncouplers are permeable throgh the plasma membrane of living cells (ce); measurement of ET-capacity.
- 4ceRotAma: Rotenone and antimycin A; measurement of residual oxygen consumption of living cells (ce) after inhibition of CI and CIII.
Further information
Cited by
- Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.8. https://doi.org/10.26124/mitofit:2021-0008
- Komlódi T, Cardoso LHD, Doerrier C, Moore AL, Rich PR, Gnaiger E (2021) Coupling and pathway control of coenzyme Q redox state and respiration in isolated mitochondria. Bioenerg Commun 2021.3. https://doi.org/10.26124/bec:2021-0003
- Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. Bioenerg Commun 2021.4. https://doi:10.26124/BEC:2021-0004
- Komlódi T, Schmitt S, Zdrazilova L, Donnelly C, Zischka H, Gnaiger E. Oxygen dependence of hydrogen peroxide production in isolated mitochondria and permeabilized cells. MitoFit Preprints (in prep).
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.8. https://doi.org/10.26124/mitofit:2021-0008
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1.
Labels: MiParea: Respiration, Instruments;methods
Organism: Mouse
Tissue;cell: Blood cells
Preparation: Permeabilized cells, Permeabilized tissue, Intact cells
Regulation: Coupling efficiency;uncoupling, Uncoupler Coupling state: LEAK, ROUTINE, ET Pathway: ROX HRR: Oxygraph-2k, TIP2k, O2k-Protocol
O2k-Demo, O2k-Core, SUIT-003 O2 ce D009, SUIT-003, SUIT-006 General O2 pce D053, Uncoupling, BEC 2020.1, BEC 2020.2, MitoFit 2021 Dark respiration, MitoFit 2021 CoQ, MitoFit 2021 AmR-O2, MitoFit 2021 AmR, MitoFit 2021 BCA, PLoSONE2022ace-sce, MitoFit2022QC