Difference between revisions of "MitoEAGLE blood cells 1"
m |
|||
| Line 7: | Line 7: | ||
|journal=MitoEAGLE | |journal=MitoEAGLE | ||
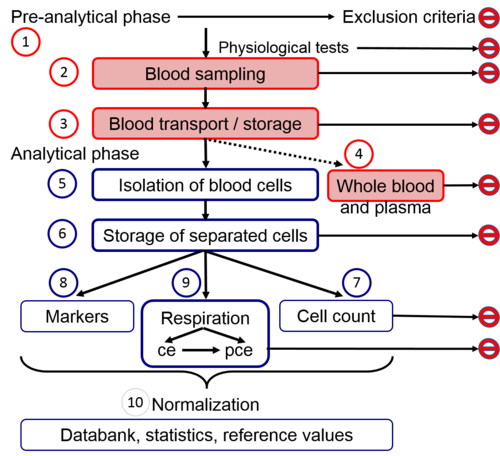
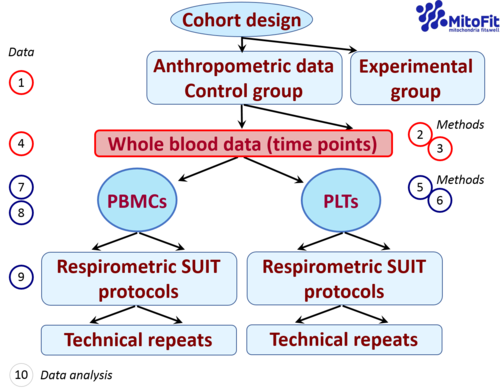
|abstract=[[File:Workflow_MitoEAGLE blood cells.png|500px|right|thumb|Fig. 1. Workflow and topics. SUIT protocols for measurement of respiration in living cells (viable cells, vce; dead cells, dce) and permeabilized cells (pce).]] | |abstract=[[File:Workflow_MitoEAGLE blood cells.png|500px|right|thumb|Fig. 1. Workflow and topics. SUIT protocols for measurement of respiration in living cells (viable cells, vce; dead cells, dce) and permeabilized cells (pce).]] | ||
Contents | |||
1. Introduction | |||
2. Methods: respiration with living and permeabilized blood cells | |||
3. Workflow: pre-analytical and analytical preparatory phase | |||
3.1. Exclusion criteria and confounding factors | |||
3.2. Blood sampling | |||
3.3. Blood transport and storage | |||
3.4. Whole blood and plasma: complementary information and exclusion criteria | |||
3.5. Isolation of blood cells | |||
3.6. Storage of isolated cells | |||
3.7. Cell count, volume, purity and viability | |||
4.1 Effect of incubation media on mitochondrial respiratory control | |||
4.2. Effect of sex and age on blood cell respiration | |||
4.3 Normalization of cell and mitochondrial respiration | |||
5. Conclusions | |||
}} | |||
__TOC__ | |||
::: '''Version beta 01''' | ::: '''Version beta 01''' | ||
:::::# The evaluation of mitochondrial function remains crucial for the diagnosis of a spectrum of human pathologies. Peripheral blood provides an easily accessible source of primary human cells. However, mitochondrial respiratory studies in blood cells still require standardization. The procedures applied in bioenergetic assessments involve multiple steps in the pre-analytical, analytical and data analysis phase. The aim of this review is to summarize methods of preparing and applying peripheral blood mononuclear cells (PBMC) and platelets (PLT) for mitochondrial respiratory studies. For this purpose we report original data from several laboratories and literature data. | :::::# The evaluation of mitochondrial function remains crucial for the diagnosis of a spectrum of human pathologies. Peripheral blood provides an easily accessible source of primary human cells. However, mitochondrial respiratory studies in blood cells still require standardization. The procedures applied in bioenergetic assessments involve multiple steps in the pre-analytical, analytical and data analysis phase. The aim of this review is to summarize methods of preparing and applying peripheral blood mononuclear cells (PBMC) and platelets (PLT) for mitochondrial respiratory studies. For this purpose we report original data from several laboratories and literature data. | ||
| Line 17: | Line 37: | ||
:::::# Normalization is important to interpret and compare the results of respiratory studies. It should include data on contamination of PBMC with PLT, cell viability (''e.g.'', succinate test), flux control ratios, total protein concentration, citrate synthase activity. Some other markers, like clusters of differentiation specific for leukocytes and platelets can be taken into consideration. | :::::# Normalization is important to interpret and compare the results of respiratory studies. It should include data on contamination of PBMC with PLT, cell viability (''e.g.'', succinate test), flux control ratios, total protein concentration, citrate synthase activity. Some other markers, like clusters of differentiation specific for leukocytes and platelets can be taken into consideration. | ||
:::::# Finally, we summarize emerging recommendations for mitochondrial respiratory studies in living and permeabilized PBMC and PLT towards building a data base of healthy subjects that can be used as controls in clinical studies. | :::::# Finally, we summarize emerging recommendations for mitochondrial respiratory studies in living and permeabilized PBMC and PLT towards building a data base of healthy subjects that can be used as controls in clinical studies. | ||
== Work in progress == | == Work in progress == | ||
Revision as of 16:57, 28 February 2020
| News and Events | Working Groups | Short-Term Scientific Missions | Management Committee | Members |
COST Action CA15203 (2016-2021): MitoEAGLE
Evolution-Age-Gender-Lifestyle-Environment: mitochondrial fitness mapping
MitoEAGLE blood cells 1
| Aasander Frostner Eleonor*, Avram Vlad F*, Calabria Elisa, Castelo Rueda Maria P, Chamkha Imen, Cizmarova Beata, Danila Maria-Daniela, Duicu Oana M, Eckert Gunter P, Ehinger Johannes K, Elmer Eskil, Engin Ayse B, Garcia-Souza Luiz F*, Gnaiger Erich*, Hoppel Florian, Karabatsiakis Alexander*, Keppner Gloria, Kidere Dita, Krako Jakovljevic Nina, Labieniec-Watala Magdalena*, Lelcu Theia, Micankova Petra, Michalak Slawomir*, Molina Anthony JA, Pavlovic Kasja, Pichler Irene, Piel Sarah, Rousar Tomas, Rybacka-Mossakowska Joanna, Schartner Melanie, Siewiera Karolina*, Silaidos Carmina, Sjoevall Fredrik, Sobotka Ondrej*, Sumbalova Zuzana*, Swiniuch Daria, Volani Chiara*, Vujacic-Mirski Ksenija, Watala Cezary* (2020) Interlaboratory guide to mitochondrial respiratory studies with peripheral blood mononuclear cells and platelets. - Updated: 2020-02-27 - *Confirmed |
» MitoEAGLE blood cells group (WG 4)
Aasander Frostner Eleonor, Avram Vlad F, Blazkova Petra, Calabria Elisa, Castelo Rueda Maria P, Chamkha Imen, Cizmarova Beata, Danila Maria-Daniela, Duicu Oana M, Eckert Gunter P, Ehinger Johannes K, Elmer Eskil, Engin Ayse B, Garcia-Souza Luiz F, Gnaiger Erich, Hoppel Florian, Karabatsiakis Alexander, Keppner Gloria, Kidere Dita, Krako Jakovljevic Nina, Labieniec-Watala Magdalena, Lelcu Theia, Michalak Slawomir, Molina Anthony JA, Pavlovic Kasja, Pichler Irene, Piel Sarah, Rousar Tomas, Rybacka-Mossakowska Joanna, Schartner Melanie, Siewiera Karolina, Silaidos Carmina, Sjoevall Fredrik, Sobotka Ondrej, Sumbalova Zuzana, Swiniuch Daria, Volani Chiara, Vujacic-Mirski Ksenija, Watala Cezary (2020) MitoEAGLE
Abstract:
Contents
1. Introduction 2. Methods: respiration with living and permeabilized blood cells 3. Workflow: pre-analytical and analytical preparatory phase 3.1. Exclusion criteria and confounding factors 3.2. Blood sampling 3.3. Blood transport and storage 3.4. Whole blood and plasma: complementary information and exclusion criteria 3.5. Isolation of blood cells 3.6. Storage of isolated cells 3.7. Cell count, volume, purity and viability 4.1 Effect of incubation media on mitochondrial respiratory control 4.2. Effect of sex and age on blood cell respiration 4.3 Normalization of cell and mitochondrial respiration 5. Conclusions
- Version beta 01
- The evaluation of mitochondrial function remains crucial for the diagnosis of a spectrum of human pathologies. Peripheral blood provides an easily accessible source of primary human cells. However, mitochondrial respiratory studies in blood cells still require standardization. The procedures applied in bioenergetic assessments involve multiple steps in the pre-analytical, analytical and data analysis phase. The aim of this review is to summarize methods of preparing and applying peripheral blood mononuclear cells (PBMC) and platelets (PLT) for mitochondrial respiratory studies. For this purpose we report original data from several laboratories and literature data.
- We do not present results on patients, but characterize healthy control groups. Exclusion criteria in the pre-analytical phase are related to smoking, alcohol intake, BMI, lifestyle interventions, and medications. In pathophysiological studies, subjects have to be matched with controls for sex, age and genetic background. Time of sampling, fasting and resting state require standardization.
- Anticoagulants are used for whole blood sampling (e.g., K-EDTA, lithium heparin, sodium citrate). Protocols are optimized on the basis of yield, cell fraction purity and respiratory function.
- The time and temperature during transport and storage of whole blood has to be monitored carefully and evaluated in terms of final outcome. Characterization of whole blood provides rigorous exclusion criteria for controls.
- Separation procedures depend on the type of cells harvested for respirometric studies (PLT, PBMC, or both). Media, centrifugation conditions, cell counting and cell viability methods are evaluated. Results on purity of preparations (e.g., PLT contamination in PBMC fraction, purity of PLT fraction), recovery and yield of cells are compared in studies using a variety of cell separation methods.
- Information is lacking on the effect of storage and conditions during processing of isolated cell types before respirometry. Temperature, media, antibiotics, proteinase inhibitors cocktail, density of cells during storage, tilting of cell fraction might significantly affect the measurements.
- Specific protocols are used for respiration assessments in living and permeabilized. The type of medium used for respirometry (MiR05, RPMI, plasma) needs further evaluation.
- Normalization is important to interpret and compare the results of respiratory studies. It should include data on contamination of PBMC with PLT, cell viability (e.g., succinate test), flux control ratios, total protein concentration, citrate synthase activity. Some other markers, like clusters of differentiation specific for leukocytes and platelets can be taken into consideration.
- Finally, we summarize emerging recommendations for mitochondrial respiratory studies in living and permeabilized PBMC and PLT towards building a data base of healthy subjects that can be used as controls in clinical studies.
- Version beta 01
Work in progress
- To summarize in a first section the respirometric tests applied for functional evaluation of methods presented in steps 2 to 7.
- The methods applied in each of the steps 2 to 7 will be introduced and compared, and directly evaluated by presenting and discussing evaluation on the basis of respirometric results.
- Cell viability and cell purity will be an outcome that can be used independently of respiration to compare and evaluate the different methods in steps 2 to 7.
- A short final summary will be presented on respirometric results (steps 8-10).
- We aim at presenting summary figures and tables with reference to original publications.
- Summary figures and tables including unpublished data are included, if the data are made available (Supplement or accessible database). Raw data should be saved on your server, then collected on a server of the corresponding author, with a statement in our manuscript that raw data are available upon request from the corresponding author (DatLab files and Excel spreadsheets).
Manuscript table and figure blocks
- Section: Workflow # and Title
- Summary: Aims [1] and recommendations
- Specify study group [2], consider sensitive data
- Methods
- Describe results shown in table and figure, as median and inter-quartile range; provide text with full information
- Discussion
- Conclusions, provide full information
- References
- (1) Define the aim, the question:
- Scientific: focus on accuracy of best physiological data, separate isolation proocols for PBMC and PLT, if necessary.
- Diagnostic: discriminative tool relying on reproducibility, practicability and minimum available blood volume; if necessary, accept compromises when PBMC and PLT should be analyzed from the same blood sample.
- (1) Define the aim, the question:
- (2) Include patient data solely for messages on methods (comparison of the effect of anticoagulants, ..); patient data can be reduced to showing fractional effects of methodological variations.
Templates for PBMC and PLT data base
- 2018-12-18 An updated template for the PBMC and PLT data base is in preparation (Luiz, Caro, Erich).
- Luiz will be responsible for collecting and merging the data sheets based on previous templates, summarizing all data in the new template.
- This will be the basis of the WG4 Task Group meeting in March 2019. We have to fix the dates.
- » MitoEAGLE template PLT final.xlsx
- » MitoEAGLE template PBMC final.xlsx
- We will collect and analyze all data, in preparation of a database for Open Access in conjunction with the journal publication.
References
- Picked up
- Zinc content of cellular components of blood: methods for cell separation and analysis evaluated. - http://clinchem.aaccjnls.org/content/31/1/65.short
- Picked up
Open questions
- How does PLT activation influence ROUTINE, LEAK and ET capacity?
- How is PBMC activation quantified and controlled, and how does it influence ROUTINE, LEAK and ET capacity?
- How does 2-3 mM lactate influence respiration in MiR05, compared to 10 mM pyruvate?
Milestones
- In progress (2018)
- March 12: Edit template of MitoEAGLE blood cell data sheet and post on this website, send alert to co-authors (Erich, Zuzana).
- March 12: Lund workshop circular (Eleonor / Marija) - invite further groups: Elisa Calabria, Kathrin Renner, Alexander Karabatriakis, Irene Pichler (contact US: Anthony Molina, Brian Irvin).
- March 15: Prepare document on regulations for sensitive data (Chiara; waiting for expert-meeting)
- March 16: Suggest topics for STSM to generate experimental data blocks for this manuscript (send to Magda).
- March 16-31: Send reminder to fill MitoEAGLE blood cell data sheets and send to Innsbruck, specify 'Open Access' (MitoEAGLE data bank on wiki), or to be shared with co-authors.
- April 1-30: Send reminder to write Table and Figure blocks and send to Innsbruck.
- April 1st week: Meeting with Anthony Molina in Innsbruck.
- April 30: MitoEAGLE workprint Version 01 - manuscript blocks arranged in work flow.
- » May 28-30: MitoEAGLE Lund 2018
- Achieved (2019)
- » Mar 11-14: MitoEAGLE WG4 Matrei a. Brenner 2019-03
- Achieved (2018)
- » May 28-30: MitoEAGLE Lund 2018
- » Feb 21-23: MitoEAGLE Poznan 2018
- » Jan 29-Feb 1: MitoEAGLE Innsbruck 2018
Publication
- A Working Group of the COST Action MitoEAGLE is preparing a manuscript 'Interlaboratory guide through procedures for mitochondrial respiratory studies with living and permeabilized peripheral blood mononuclear cells and platelets'. The group is working on it with Open Access as a ‘MitoEAGLE preprint’ and the ultimate aim of publication in a scientific journal.
- Compare: Gnaiger 2019 MitoFit Preprint Arch
Co-authors
- The present alphabetical list of co-authors icludes all actively involved participants of MitoEAGLE Innsbruck 2018 and MitoEAGLE Poznan 2018 and will be extended by further contributors at MitoEAGLE Lund 2018 and by MitoEAGLE members submitting their valuable contributions. All co-authors will have to confirm to have made a contribution and to have read the final manuscript.
Publication strategy
- Data linked to published papers or manuscripts in press are superior to unpublished data.
- Inclusion of unpublished data should not block independent publication by the indivudual teams.
- Data classified as occasional observations or preliminary results are statistically valuable when combined with comparable data from other groups - pull out your unpublished observations (data).
- Acknowledgements: We thank M. Beno for management assistance. This publication is based upon work from COST Action CA15203 MitoEAGLE, supported by COST (European Cooperation in Science and Technology), and K-Regio project MitoFit (E.G.).
Labels: MiParea: Respiration, Instruments;methods, mtDNA;mt-genetics, nDNA;cell genetics, Gender
Pathology: Aging;senescence
Organism: Human Tissue;cell: Blood cells, Platelet Preparation: Intact cells, Permeabilized cells Enzyme: Marker enzyme
Coupling state: LEAK, ROUTINE, OXPHOS, ET Pathway: F, N, S, Gp, CIV, NS, Other combinations, ROX
MitoFitPublication, MitoEAGLEPublication