Description
Abbreviation: FNS(Oct,GM)
Reference: A pfi: permeabilized fibers- SUIT-016
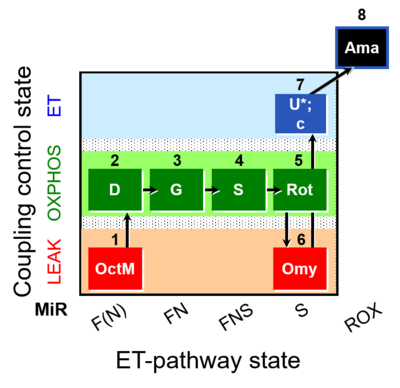
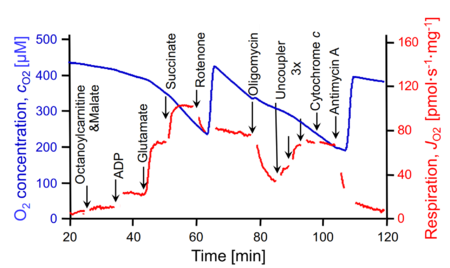
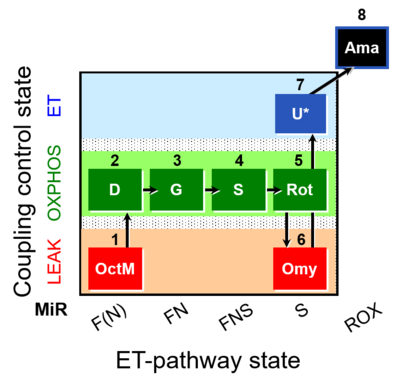
SUIT number: D044_1OctM;2D;3G;4S;5Rot;6Omy;7U;8Ama
O2k-Application: O2
- SUIT-category: FNS(Oct,PGM)
- SUIT protocol pattern: diametral 1OctM;2D;3G;4S;5Rot;6Omy;7U;8Ama
Communicated by Cardoso LH and Gnaiger E (last update 2019-02-14)
Steps and respiratory states
| Step | State | Pathway | Q-junction | Comment - Events (E) and Marks (M) |
|---|---|---|---|---|
| 1OctM | OctML(n) | F(N) | CETF | 1OctM
|
| 2D | OctMP | F(N) | CETF | 1OctM;2D
|
| 3G | OctGMP | FN | CETF&I | 1OctM;2D;3G
|
| 4S | OctGMSP | FNS | CETF&CI&II | 1OctM;2D;3G;4S
|
| 5Rot | SP | S | CII | 1OctM;2D;3G;4S;5Rot
|
| 6Omy | SL(Omy) | S | CII | 1OctM;2D;3G;4S;5Rot;6Omy
|
| 7U | SE | S | CII | 1OctM;2D;3G;4S;5Rot;6Omy;7U
|
| 8Ama | ROX | 1OctM;2D;3G;4S;5Rot;6Omy;7U;8Ama
|
| Step | Respiratory state | Pathway control | ET-Complex | Comment |
|---|---|---|---|---|
| ## AsTm | AsTmE | CIV | CIV | |
| ## Azd | CHB |
- Bioblast links: SUIT protocols - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coupling control
- Pathway control
- Main fuel substrates
- » Glutamate, G
- » Glycerophosphate, Gp
- » Malate, M
- » Octanoylcarnitine, Oct
- » Pyruvate, P
- » Succinate, S
- Main fuel substrates
- Glossary
Strengths and limitations
- + This protocol provides information on FAO capacity in the absence of other potentially interfering pathways both in LEAK and OXPHOS coupling control states.
- + FNS OXPHOS capacity comprises the most important pathways in many cell types and thus provides a physiologically relevant estimate of maximum mitochondrial respiratory capacity.
- + FNS ET capacity is a good estimate of overall ET capacity in many cell types.
- + This is a good protocol to analyse coupling control at S-pathway, which is an advantage compared to SUIT-015 and SUIT-017.
- + Glutamate is easier to prepare compared to pyruvate.
- - In some tissues GM is not sufficient to fully support N-pathway capacity
- - SRot(E) may be underestimated if S is not saturating.
- - SRot(E) may be underestimated in the presence of Omy in some tissues and cell types
- - CIV activity is not measured, to save experimental time.
- It is possible to add cytochrome c in different steps:
- (2c), when cytochrome c is added after 2D, it allows to compare all steps in OXPHOS even if there is damage of the mitochondrial outer membrane, and can be chosen when the cytochrome c effect is not an exclusion criteria.
- (3c) when cytochrome c is added after 3G, due to the higher flux, it is easier to notice whether there is an increase in respiration after addition of cytochrome c. However, if there is a strong cytochrome c effect, it is not possible to compare OctMP with the next steps in OXPHOS.
- (7c) when cytochrome c is added after 7U, can be used as an exclusion criteria.
Compare SUIT protocols
References
| Year | Reference | Organism | Tissue;cell | |
|---|---|---|---|---|
| Gnaiger 2015 Scand J Med Sci Sports | 2015 | Gnaiger E, Boushel R, Søndergaard H, Munch-Andersen T, Damsgaard R, Hagen C, Díez-Sánchez C, Ara I, Wright-Paradis C, Schrauwen P, Hesselink M, Calbet JAL, Christiansen M, Helge JW, Saltin B (2015) Mitochondrial coupling and capacity of oxidative phosphorylation in skeletal muscle of Inuit and caucasians in the arctic winter. https://doi.org/10.1111/sms.12612 | Human | Skeletal muscle |
MitoPedia concepts: SUIT protocol, SUIT A, Find
MitoPedia methods:
Respirometry