Doerrier 2017 MiPschool C2
| Substrate-uncoupler-inhibitor titration (SUIT) protocols – fundamental principles. |
Link: MitoEAGLE
Doerrier C (2017)
Event: MiPschool Obergurgl 2017
A wide diversity of substrate-uncoupler-inhibitor titration (SUIT) protocols has been used for evaluation of mitochondrial respiration in different samples and under various experimental conditions [1]. We developed a SUIT reference protocol (RP) with the goal to provide a common reference for establishing a database on comparative mitochondrial physiology [2]. The SUIT-RP consists of two complementary SUIT protocols: SUIT-RP1 [3] and SUIT-RP2 [4]. These protocols comprise specific respiratory pathways (FNSGp) converging at the Q-junction (Figure 1). RP1 and RP2 are harmonized by inclusion of two comparable respiratory states (SGpE and CIVE). Harmonized states differ in terms of the sequence of substrate, uncoupler and inhibitor titrations (Figure 2). RP1 includes respiratory states that can be compared to many previously published SUIT protocols. In the present communication, we address specifically the following topics: Are ET-pathway capacities identical in the harmonized states of RP1 and RP2 applied in parallel experiments? In our updated ‘final’ version of RP2, the sequence and concentrations of malate titrations was optimized to distinguish fatty acid oxidation capacity from the N-pathway capacity. The harmonized respiratory states were in excellent agreement in a parallel experimental design with RP1 and RP2 (test and ‘final’) applied to (i) isolated mouse cardiac mitochondria, (ii) brain tissue homogenate from mice, and (iii) cryopreserved permeabilized HEK cells [5]. On the other hand, previous studies demonstrate [6] that N pathway cannot be fully isolated from S pathway. S-contribution in SUIT protocols depends on the N-substrate combinations used. We tested S-contribution in RP1 and RP2 protocols for a better understanding of bioenergetic complexity in mitochondrial physiology. In conclusion, an optimal design and comprehension of the SUIT protocols is crucial for a proper evaluation of mitochondrial function in healthy and pathological conditions.
• Bioblast editor: Doerrier_Velasco_CA
• O2k-Network Lab: AT Innsbruck Oroboros
Labels: MiParea: Respiration, Comparative MiP;environmental MiP
Stress:Cryopreservation Organism: Human, Mouse Tissue;cell: Heart, Nervous system, HEK Preparation: Permeabilized cells, Homogenate, Isolated mitochondria
Coupling state: LEAK, ROUTINE, OXPHOS, ET
Pathway: F, N, S, Gp, CIV
HRR: Oxygraph-2k, O2k-Fluorometer
Event: C1, Review
Affiliations
- Oroboros Instruments, Innsbruck, AT. - [email protected]
Figure 1
Figure 1. Multiple ET pathways with FNSGpCIV. Schematic representation of multiple electron transfer-pathway (ET-pathway) pathways. Electrons converge at the Q-junction from Complex I (CI) or NADH (N)-pathway, Complex II (CII) or Succinate (S)-pathway, glycerophosphate dehydrogenase Complex (CGpDH) or Gp-pathway, electron-transferring flavoprotein Complex (CETF) or F-pathway. Electron flow follows by a linear downstream segment through Complexes III (CIII, ubiquinol-cytochrome c oxidoreductase) and CIV (cytochrome c oxidase), to the final electron acceptor oxygen.
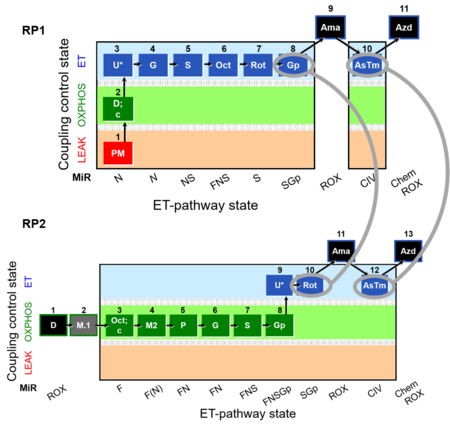
Figure 2
Figure 2. Reference protocols: RP1 and RP2. Harmonization between RP1 and RP2. RP1) RP1 which starts with linear coupling control, L – P - E, with the type N substrates pyruvate and malate (N-pathway to Q), thus separating coupling control and the subsequent linear sequence of pathway control in the ET-pathway state. RP2) RP2 has a focus on OXPHOS capacity of fatty acid oxidation (F-pathway) compared to OXPHOS capacity with combined FN- (FN-pathways to Q) and FNS- (FNS-pathways to Q) type substrates. RP2 adds a sequence of pathway control steps to measure maximum OXPHOS and ET capacity with a FNSGp substrate combination to activate pathways converging at the Q-junction through Complex I (CI), electron-transferring flavoprotein complex (CETF), Complex II (CII), and glycerophosphate dehydrogenase complex (CGpDH). Finally, RP1 and RP2 provide information on the activity of the single enzyme step of Complex IV (CIV) downstream of Q.
Support
References
- MitoPedia:_SUIT - Substrate-uncoupler-inhibitor protocols.
- MiPNet21.06_SUIT_RP – SUIT reference protocol for OXPHOS analysis by high-resolution respirometry.
- 1PM;2D;3U;4G;5S;6Oct;7Rot;8Gp- – Reference protocol 1 (RP1).
- 1D;2OctM;3P;4G;5S;6Gp;7U;8Rot- – Reference protocol 2 (RP2).
- MiPNet21.14_Reference_sample_HRR – Development of a reference sample for HRR.
- Sumbalova_2016a_Abstract_MitoFit_Science_Camp_2016 – Optimizing strategies on the malate concentration in SUIT protocols.