Gnaiger 2015 Abstract MiPschool Cape Town 2015
| The functional design of the electron transfer system (ETS) and mitochondrial respiratory control. |
Link:
Gnaiger E (2015)
Event: MiPschool Cape Town 2015
Mitochondrial (mt) respiration is the fundamental bioenergetic process of oxidative phosphorylation in aerobic cells, which is vitally important for health, physical fitness and protective medicine. In oxidative phosphorylation (OXPHOS), electron transfer from reduced substrates to oxygen drives the mt-membrane potential, Δpmt, through the proton pumps of respiratory Complexes CI, CIII and CIV. Cell respiration thus depends on and is strongly controlled by a continuous flow of fuel substrates and products across the mt-membranes between the matrix and cytosolic space. Some substrates are transported across plasma and mt-membranes (e.g. H+/monocarboxylate cotransport for pyruvate). In contrast, the dicarboxylic acid succinate, which is formed in the tricarboxylic acid (TCA) cycle in the mt-matrix, is not transported across the plasma membrane of many cell types, but is readily exchanged across the inner mt-membrane. The detailed study of substrate control of mitochondrial respiration, therefore, requires selective cell membrane permeabilization in mt-preparations (permeabilized cells and tissues, homogenates, isolated mitochondria). The electron transfer-pathway (ET) capacity of mt-preparations depends on (i) substrate type and substrate transport across mt-membranes, (ii) TCA cycle and other mt-matrix dehydrogenase activities, and (iii) activity of the inner mt-membrane bound ETS (mETS) [1].
Functional studies of mt-preparations include measurement of respiration, ATP production (P»), mt-membrane potential and ROS production, which require substrates to be applied and enzymes to be inhibited selectively to differentiate distinct mt-pathways, following two different strategies.
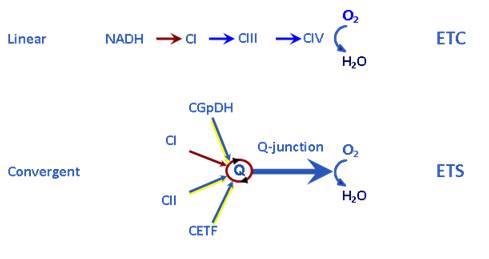
(i) Physiological pathway control states are designed to reconstitute TCA cycle function of the intact cell, to quantify physiological ET- and OXPHOS-capacities. Multiple substrates are supplied to counterbalance intermediary metabolite loss from the mitochondria into the dilute incubation medium. Physiological combinations of NADH-linked substrates (N-linked: pyruvate&glutamate&malate, PGM) and succinate (S) enhance ET-capacity due to the additive effect of convergent electron transfer at the Q-junction (Fig. 1). The ETS segment CI-CIII-CIV is organized as a supercomplex to various degrees [2]. Tight electron channeling through supercomplexes prevents lateral electron exchange between ETS redox components, maximizes the additive effect of multiple substrates, and reduces mt-ROS production by electron leaks. Whereas the ETS segment CI-CIII-CIV generates very low levels of ROS, the CII-CIII-CIV segment is currently not considered as a supercomplex (but compare [2]) with high electron leak to ROS when studied as a separate pathway (see ii). The glycerophosphate dehydrogenase complex (CGpDH) and electron-transferring flavoprotein complex of fatty acid oxidation (CETF) provide additional convergent electron entries into the Q-junction (Fig. 1), without known supercomplex design, with lower additivity for oxygen flux but high contributions to ROS production.
• O2k-Network Lab: AT Innsbruck Oroboros
Abstract continued
(ii) In linear pathway states convergent electron entry is blocked and restricted to a single branch of the ETS pathways. Under these conditions of electron gating, control of ET-capacity is exerted mainly by substrate transport into the mitochondria, specific inhibitors applied to block selected mt-pathways, metabolite depletion from the mt-matrix, and activities of mt-matrix dehydrogenases. Electron gating restricts ET-capacity and shifts flux control artificially upstream of the Q-junction. This provides diagnostic information on specific branches of the ETS, particularly with (a) N-linked substrates (PM, GM or PGM; ETC in Fig. 1), (b) S-linked succinate while blocking CI with rotenone, or (c) fatty acid oxidation with a fatty acid (free or bound to carnitine) and malate. Respiratory flux through specific segments of the ETS can be best compared by normalization of flux as substrate control ratios [1]. Electron gating is the basis to evaluate the H+/electron stoichiometry of the proton pumps and the P»/O2 ratio in the N- versus S-pathway.
Substrate-uncoupler-inhibitor titration (SUIT) protocols are designed to obtain a maximum of diagnostic information in single incubation assays, made possible by multiple sequential titrations in high-resolution respirometry (HRR; Oroboros O2k) [3]. Numerous quantitative studies using SUIT protocols in HRR have become available during the past few years, which reveal a surprising diversity of mitochondrial respiratory control between species, tissues and cell types. This paradigm shift from unity in the biochemistry of bioenergetics to evolutionary diversity of mitochondrial respiratory function sets the foundations of a new comparative mitochondrial physiology [4]. Comprehension of the adaptive traits underlying this functional diversity in pathway control of mitochondrial respiration will be required as a basis to discriminate between mitochondrial health and disease, to develop quantitative mitochondrial fitness scores for specific tissues and cell types, and develop OXPHOS analysis further towards standardized diagnostic tests as required in biomedical studies and clinical applications.
Figures
Figure 1. Electron transfer chain (ETC, linear) versus electron transfer system (ETS, convergent). CI to CIV, Complex I to IV; CGpDH, glycerophosphate dehydrogenase complex; CETF, electron-transferring flavoprotein complex. Electron gating (ETC) limits flux upstream if convergent electron supply exerts an additive effect on ET-capacity. From Gnaiger 2014 [1].
Affiliation
- Swarovski Research Lab, Dept Visceral, Transplant Thoracic Surgery, Medical Univ Innsbruck;
- OROBOROS INSTRUMENTS, Innsbruck, Austria. - [email protected]
References and acknowledgements
- Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Mitochondr Physiol Network 19.12. Oroboros MiPNet Publications, Innsbruck:80 pp.
- Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the electron transfer-pathway XLII. Reconstitution of the electron transfer-pathway. J Biol Chem 237:2661-9.
- Pesta D, Gnaiger E (2012) High-resolution respirometry. OXPHOS protocols for human cells and permeabilized fibres from small biopsies of human muscle. Meth Mol Biol 810:25-58.
- Laner V, Bidaurratzaga E, Gnaiger E, eds (2013) Comparative mitochondrial physiology. MiP2013. Mitochondr Physiol Network 18.08:96 pp.
Labels: MiParea: Respiration, mt-Membrane, Comparative MiP;environmental MiP, Exercise physiology;nutrition;life style, mt-Medicine, mt-Awareness
Stress:Oxidative stress;RONS
Preparation: Permeabilized cells, Permeabilized tissue, Homogenate, Isolated mitochondria
Enzyme: Supercomplex
Regulation: Substrate
Coupling state: LEAK, ROUTINE, OXPHOS, ET
Pathway: N, S, NS
HRR: Oxygraph-2k