Difference between revisions of "Fink 2018 J Biol Chem"
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Publication | {{Publication | ||
|title=Fink BD, Bai F, Yu L, Sheldon RD, Sharma A, Taylor EB, Sivitz WI (2018) Oxaloacetic acid mediates ADP-dependent inhibition of mitochondrial complex II-driven respiration. J Biol Chem 293:19932-41. | |title=Fink BD, Bai F, Yu L, Sheldon RD, Sharma A, Taylor EB, Sivitz WI (2018) Oxaloacetic acid mediates ADP-dependent inhibition of mitochondrial complex II-driven respiration. J Biol Chem 293:19932-41. https://doi.org/10.1074/jbc.RA118.005144 | ||
|info=[https://www.ncbi.nlm.nih.gov/pubmed/30385511 PMID: 30385511 Open Access] | |info=[https://www.ncbi.nlm.nih.gov/pubmed/30385511 PMID: 30385511 Open Access] | ||
|authors=Fink BD, Bai F, Yu L, Sheldon RD, Sharma A, Taylor EB, Sivitz WI | |authors=Fink BD, Bai F, Yu L, Sheldon RD, Sharma A, Taylor EB, Sivitz WI | ||
| Line 10: | Line 10: | ||
|mipnetlab=US IA Iowa City Sivitz WI, US MO Columbia Rector RS | |mipnetlab=US IA Iowa City Sivitz WI, US MO Columbia Rector RS | ||
}} | }} | ||
[[File:Fink 2018 J Biol Chem CORRECTION.png|right|400px]] | |||
{{Template:Correction FADH2 and S-pathway}} | |||
{{Labeling | {{Labeling | ||
|area=Respiration | |area=Respiration | ||
| Line 19: | Line 22: | ||
|couplingstates=OXPHOS | |couplingstates=OXPHOS | ||
|pathways=N, S, NS | |pathways=N, S, NS | ||
|instruments=Oxygraph-2k | |instruments=Oxygraph-2k, TPP | ||
|additional= | |additional=2018-11, | ||
}} | }} | ||
Latest revision as of 01:16, 3 April 2023
| Fink BD, Bai F, Yu L, Sheldon RD, Sharma A, Taylor EB, Sivitz WI (2018) Oxaloacetic acid mediates ADP-dependent inhibition of mitochondrial complex II-driven respiration. J Biol Chem 293:19932-41. https://doi.org/10.1074/jbc.RA118.005144 |
Fink BD, Bai F, Yu L, Sheldon RD, Sharma A, Taylor EB, Sivitz WI (2018) J Biol Chem
Abstract: We recently reported a previously unrecognized mitochondrial respiratory phenomenon. When [ADP] was held constant ("clamped") at sequentially increasing concentrations in succinate-energized muscle mitochondria in the absence of rotenone (commonly used to block complex I), we observed a biphasic, increasing then decreasing, respiratory response. Here we investigated the mechanism. We confirmed decades old reports that oxaloacetate (OAA) inhibits succinate dehydrogenase (SDH). We then used an NMR method to assess OAA concentrations (known as difficult to measure by mass spectroscopy) as well as those of malate, fumarate, and citrate in isolated succinate-respiring mitochondria. When these mitochondria were incubated at varying clamped ADP concentrations, respiration increased at low [ADP], as expected given the concurrent reduction in membrane potential. With further increments in [ADP], respiration decreased associated with accumulation of OAA. Moreover, a low pyruvate concentration, that alone was not enough to drive respiration, was sufficient to metabolize OAA to citrate and completely reverse the loss of succinate-supported respiration at high [ADP]. Further, chemical or genetic inhibition of pyruvate uptake prevented OAA clearance and preserved respiration. In addition, we measured the effects of incremental [ADP] on NADH, superoxide, and H2O2 (a marker of reverse electron transport from complex II to I). In summary, our findings, taken together, support a mechanism (detailed within) wherein succinate-energized respiration as a function of increasing [ADP] is initially increased by [ADP] dependent effects on membrane potential but subsequently decreased at higher [ADP] by inhibition of SDH by OAA. The physiologic relevance is discussed. • Keywords: ADP, ATP, Bioenergetics, Mitochondria, Mitochondrial metabolism, Mitochondrial respiratory chain complex, Nuclear magnetic resonance (NMR), Oxaloacetate, Skeletal muscle, Succinate, Succinate dehydrogenase • Bioblast editor: Plangger M • O2k-Network Lab: US IA Iowa City Sivitz WI, US MO Columbia Rector RS
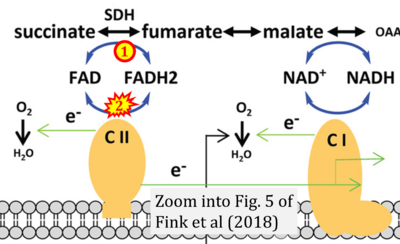
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: MiParea: Respiration
Organism: Mouse
Tissue;cell: Skeletal muscle
Preparation: Isolated mitochondria
Enzyme: Complex II;succinate dehydrogenase
Regulation: ADP, Substrate
Coupling state: OXPHOS
Pathway: N, S, NS
HRR: Oxygraph-2k, TPP
2018-11