Zhang 2018 Mil Med Res
| Zhang H, Feng YW, Yao YM (2018) Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil Med Res 5:41. https://doi.org/10.1186/s40779-018-0187-0 |
Zhang Hui, Feng Yong-Wen, Yao Yong-Ming (2018) Mil Med Res
Abstract: Recently, the definition of sepsis was concluded to be a life-threatening organ dysfunction caused by a dysregulated host response to infection. Severe patients always present with uncorrectable hypotension or hyperlactacidemia, which is defined as septic shock. The new definition emphasizes dysregulation of the host response and multiple organ dysfunction, which is partially attributed to metabolic disorders induced by energy crisis and oxidative stress. Mitochondria are a cellular organelle that are well known as the center of energy production, and mitochondrial damage or dysfunction is commonly induced in septic settings and is a predominant factor leading to a worse prognosis. In the present review, we determine the major mitochondrial disorders from morphology to functions in sepsis. In the following, several clinical or pre-clinical assays for monitoring mitochondrial function are demonstrated according to accumulated evidence, which is the first step of specific therapy targeting to modulate mitochondrial function. Accordingly, various reagents used for regulating mitochondrial enzyme activities and promoting biogenesis have been documented, among which mitochondria-targeted cation, TPP-conjugated antioxidants are the most valuable for future trials and clinical treatment to improve mitochondrial function as they may take advantage of the prognosis associated with septic complications. • Keywords: Electron transfer chain, Mitochondria, Monitor, Sepsis, Therapy strategy • Bioblast editor: Plangger M
Labels: MiParea: Respiration
Pathology: Sepsis
Tissue;cell: Blood cells, Platelet
HRR: Oxygraph-2k
Labels, 2019-12, CN, MitoEAGLE blood cells reviews
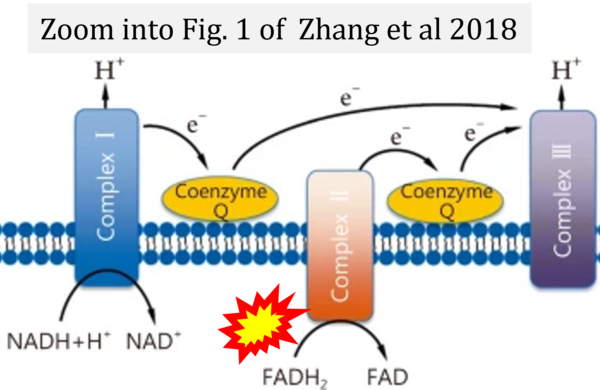
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«