Shirakawa 2023 Sci Rep
| Shirakawa R, Nakajima T, Yoshimura A, Kawahara Y, Orito C, Yamane M, Handa H, Takada S, Furihata T, Fukushima A, Ishimori N, Nakagawa M, Yokota I, Sabe H, Hashino S, Kinugawa S, Yokota T (2023) Enhanced mitochondrial oxidative metabolism in peripheral blood mononuclear cells is associated with fatty liver in obese young adults. https://doi.org/10.1038/s41598-023-32549-w |
» Sci Rep 13:5203. PMID: 36997629 Open Access
Shirakawa R, Nakajima T, Yoshimura A, Kawahara Y, Orito C, Yamane M, Handa H, Takada S, Furihata T, Fukushima A, Ishimori N, Nakagawa M, Yokota I, Sabe H, Hashino S, Kinugawa S, Yokota T (2023) Sci Rep
Abstract: Systemic inflammation underlies the association between obesity and nonalcoholic fatty liver disease (NAFLD). Here, we investigated functional changes in leukocytes' mitochondria in obese individuals and their associations with NAFLD. We analyzed 14 obese male Japanese university students whose body mass index was > 30 kg/m2 and 15 healthy age- and sex-matched lean university students as controls. We observed that the mitochondrial oxidative phosphorylation (OXPHOS) capacity with complex I + II-linked substrates in peripheral blood mononuclear cells (PBMCs), which was measured using a high-resolution respirometry, was significantly higher in the obese group versus the controls. The PBMCs' mitochondrial complex IV capacity was also higher in the obese subjects. All of the obese subjects had hepatic steatosis defined by a fatty liver index (FLI) score ≥ 60, and there was a positive correlation between their FLI scores and their PBMCs' mitochondrial OXPHOS capacity. The increased PBMCs' mitochondrial OXPHOS capacity was associated with insulin resistance, systemic inflammation, and higher serum levels of interleukin-6 in the entire series of subjects. Our results suggest that the mitochondrial respiratory capacity is increased in the PBMCs at the early stage of obesity, and the enhanced PBMCs' mitochondrial oxidative metabolism is associated with hepatic steatosis in obese young adults.
• Bioblast editor: Plangger M • O2k-Network Lab: JP Sapporo Yokota T
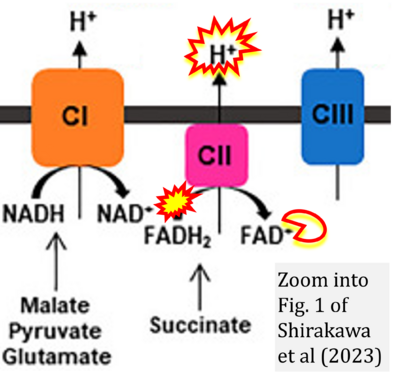
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
On terminology
- For harmonization of terminology on respiratory states and rates, see
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. https://doi.org/10.26124/bec:2020-0001.v1
- For harmonization of terminology on respiratory states and rates, see
Labels:
MitoEAGLE terminology
Labels: MiParea: Respiration, Patients Pathology: Obesity
Organism: Human Tissue;cell: Blood cells Preparation: Permeabilized cells
Regulation: ADP, Inhibitor, Substrate, Uncoupler Coupling state: LEAK, OXPHOS, ET
HRR: Oxygraph-2k
2023-04, JP