Moudgil 2005 J Appl Physiol (1985)
| Moudgil R, Michelakis ED, Archer SL (2005) Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985) 98:390-403. https://doi.org/10.1152/japplphysiol.00733.2004 |
Moudgil R, Michelakis ED, Archer SL (2005) J Appl Physiol (1985)
Abstract: Humans encounter hypoxia throughout their lives. This occurs by destiny in utero, through disease, and by desire, in our quest for altitude. Hypoxic pulmonary vasoconstriction (HPV) is a widely conserved, homeostatic, vasomotor response of resistance pulmonary arteries to alveolar hypoxia. HPV mediates ventilation-perfusion matching and, by reducing shunt fraction, optimizes systemic Po(2). HPV is intrinsic to the lung, and, although modulated by the endothelium, the core mechanism is in the smooth muscle cell (SMC). The Redox Theory for the mechanism of HPV proposes the coordinated action of a redox sensor (the proximal mitochondrial electron transport chain) that generates a diffusible mediator [a reactive O(2) species (ROS)] that regulates an effector protein [voltage-gated potassium (K(v)) and calcium channels]. A similar mechanism for regulating O(2) uptake/distribution is partially recapitulated in simpler organisms and in the other specialized mammalian O(2)-sensitive tissues, including the carotid body and ductus arteriosus. Inhibition of O(2)-sensitive K(v) channels, particularly K(v)1.5 and K(v)2.1, depolarizes pulmonary artery SMCs, activating voltage-gated Ca(2+) channels and causing Ca(2+) influx and vasoconstriction. Downstream of this pathway, there is important regulation of the contractile apparatus' sensitivity to calcium by rho kinase. Controversy remains as to whether hypoxia decreases or increases ROS and which electron transport chain complex generates the ROS (I and/or III). Possible roles for cyclic adenosine diphosphate ribose and an unidentified endothelial constricting factor are also proposed by some groups. Modulation of HPV has therapeutic relevance to cor pulmonale, high-altitude pulmonary edema, and sleep apnea. HPV is clinically exploited in single-lung anesthesia, and its mechanisms intersect with those of pulmonary arterial hypertension.
• Bioblast editor: Gnaiger E
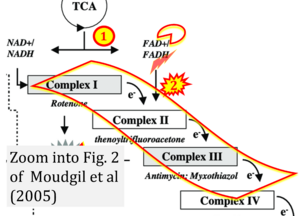
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Stress:Hypoxia
Tissue;cell: Lung;gill