Jian 2020 Cell Metab
| Jian C, Fu J, Cheng X, Shen LJ, Ji YX, Wang X, Pan S, Tian H, Tian S, Liao R, Song K, Wang HP, Zhang X, Wang Y, Huang Z, She ZG, Zhang XJ, Zhu L, Li H (2020) Low-dose sorafenib acts as a mitochondrial uncoupler and ameliorates nonalcoholic steatohepatitis. Cell Metab 31:892-908. https://doi.org/10.1016/j.cmet.2020.04.011 |
Jian Chongshu, Fu Jiajun, Cheng Xu, Shen Li-Jun, Ji Yan-Xiao, Wang Xiaoming, Pan Shan, Tian Han, Tian Song, Liao Rufang, Song Kehan, Wang Hai-Ping, Zhang Xin, Wang Yibin, Huang Zan, She Zhi-Gang, Zhang Xia-Jing, Zhu Lihua, Li Hongliang (2020) Cell Metab
Abstract: Nonalcoholic steatohepatitis (NASH) is becoming one of the leading causes of hepatocellular carcinoma (HCC). Sorafenib is the only first-line therapy for advanced HCC despite its serious adverse effects. Here, we report that at an equivalent of approximately one-tenth the clinical dose for HCC, sorafenib treatment effectively prevents the progression of NASH in both mice and monkeys without any observed significant adverse events. Mechanistically, sorafenib's benefit in NASH is independent of its canonical kinase targets in HCC, but involves the induction of mild mitochondrial uncoupling and subsequent activation of AMP-activated protein kinase (AMPK). Collectively, our findings demonstrate a previously unappreciated therapeutic effect and signaling mechanism of low-dose sorafenib treatment in NASH. We envision that this new therapeutic strategy for NASH has the potential to translate into a beneficial anti-NASH therapy with fewer adverse events than is observed in the drug's current use in HCC.
Copyright © 2020 Elsevier Inc. All rights reserved. • Keywords: AMP–activated protein kinase (AMPK), Mitochondrial uncoupler, Nonalcoholic steatohepatitis (NASH), Sorafenib • Bioblast editor: Plangger M
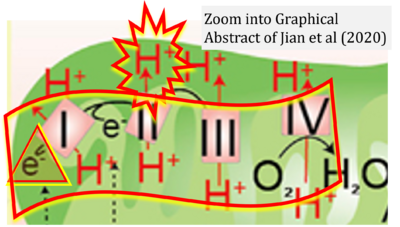
Correction: CII is not a proton pump
- While CI functions as a proton pump, CII does not. Depicting CII as a proton pump would be analogous to falsely portraying FADH2 as the substrate of CII, as if it were a copy of CI, which functions as a proton pump with NADH as its substrate. For details see Ref [1].
- Gnaiger E (2023) Complex II ambiguities ― FADH2 in the electron transfer system. MitoFit Preprints 2023.3. https://doi.org/10.26124/mitofit:2023-0003.v2
Labels: MiParea: Respiration, Pharmacology;toxicology Pathology: Cancer
Organism: Mouse Tissue;cell: Liver Preparation: Permeabilized cells
Coupling state: LEAK, OXPHOS, ET
Pathway: N, S, CIV, ROX
HRR: Oxygraph-2k
2020-05, CN