Huetter 2004 Biochem J
| Hütter E, Renner K, Pfister G, Stöckl P, Jansen-Dürr P, Gnaiger E (2004) Senescence-associated changes in respiration and oxidative phosphorylation in primary human fibroblasts. https://doi.org/10.1042/BJ20040095 |
» Biochem J 380:919-28. PMID: 15018610 - Open Access
Huetter E, Renner K, Pfister G, Stoeckl P, Jansen-Duerr Pidder, Gnaiger Erich (2004) Biochem J
Abstract: Limitation of lifespan in replicative senescence is related to oxidative stress, which is probably both the cause and consequence of impaired mitochondrial respiratory function. The respiration of senescent human diploid fibroblasts was analysed by high-resolution respirometry. To rule out cell-cycle effects, proliferating and growth-arrested young fibroblasts were used as controls. Noncoupled respiration, as normalized to citrate synthase activity, remained unchanged, reflecting a constant mitochondrial Electron transfer pathway (ET-pathway) capacity. Oligomycin-inhibited LEAK respiration, however, was significantly increased in mitochondria of senescent cells, indicating a lower coupling of electron transfer to phosphorylation of ADP to ATP. In contrast, growth-arrested young fibroblasts exhibited a higher coupling control compared with proliferating controls. In living cells, partial uncoupling (dyscoupling) may lead to either decreased oxidative ATP production or a compensatory increase in ROUTINE respiration. To distinguish between these alternatives, we subtracted oligomycin-inhibited respiration from ROUTINE respiration, which allowed us to determine the part of respiratory activity coupled with ATP production. Despite substantial differences in the respiratory acceptor control ratio, ranging from 4 to 11 in the different experimental groups, a fixed proportion of ET-capacity was maintained for coupled oxidative phosphorylation in all the experimental groups. This finding indicates that the senescent cells fully compensate for increased proton leakage by enhanced electron-transfer activity in the ROUTINE state. These results provide a new insight into age-associated defects in mitochondrial function and compensatory mechanisms in living cells. • Keywords: Aging, Coupling state, Mitochondria, Oxidative stress, Primary human fibroblast, Respiration, Senescence.
• O2k-Network Lab: AT Innsbruck Gnaiger E, AT Innsbruck Jansen-Duerr P, DE Regensburg Renner-Sattler K
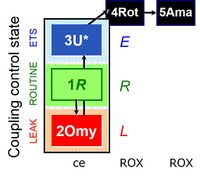
Coupling control protocol and nomenclature
- ceCCP: 1ce;2ceOmy;3ceU-
- An update on nomenclature
- State 4o »new: LEAK state with oligomycin
- State 3u, uncoupled »new: ET-capacity
- j(R-4o)/3u »new: NetROUTINE control ratio, (R-L)/E
- Ro »new: Rot
- AA »new: Ama
- An update on nomenclature
High-resolution respirometry with SUIT-003
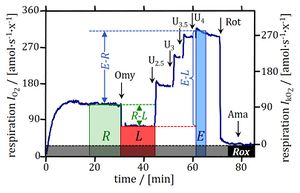
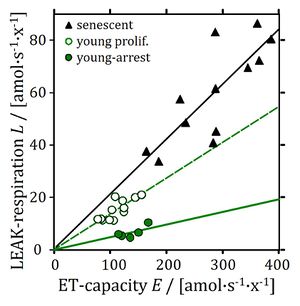
- Cell coupling control protocol: respiration in human primary fibroblasts. Left: High-resolution respirometry in senescent cells (0.2×106 x/mL). Arrows show steps in the titration regime of the coupling control protocol, inducing the following respiratory states: ROUTINE-respiration (R; routine state in cell-culture medium); LEAK-respiration (L; inhibition of ATP synthase by 1 μg/mL oligomycin); ET-capacity (E; maximal stimulation by uncoupling of oxidative phosphorylation in four subsequent titrations of the uncoupler, U, FCCP (2.5–4 μM final concentration); ROX-state (rotenone, Rot, and antimycin A; residual oxygen consumption Rox). Right: Rox-corrected oxygen flow per cell, showing different coupling control in senescent versus young cells by plotting LEAK-respiration as a function of ET-efficiency. Every data point is a single run in an O2k chamber. From Gnaiger 2020 BEC MitoPathways modified after Huetter 2004 Biochem J.
Comment
- In the context of another study (MiP2005 Abstract), we compared respiration of living (ce) and permeabilized (pce) fibroblasts with excellent results. Therefore, permeabilized cells provide a validated model to extend mitochondrial studies of senescence. In fact, the fibroblasts in the present study were permeabilized for the JC1 experiments, to eliminate the confounding effect of the plasma membrane potential.
Cited by
- 70 articles in PubMed (2021-12-27) https://pubmed.ncbi.nlm.nih.gov/15018610/
- Gnaiger E (2021) Bioenergetic cluster analysis – mitochondrial respiratory control in human fibroblasts. MitoFit Preprints 2021.8. https://doi.org/10.26124/mitofit:2021-0008
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
Labels: MiParea: Respiration, mt-Biogenesis;mt-density
Pathology: Aging;senescence
Stress:Oxidative stress;RONS
Organism: Human
Tissue;cell: Fibroblast
Preparation: Intact cells
Enzyme: Marker enzyme
Regulation: Coupling efficiency;uncoupling, mt-Membrane potential, Uncoupler
Coupling state: LEAK, ROUTINE, ET
HRR: Oxygraph-2k, O2k-Protocol
SUIT-003 O2 ce D009, SUIT-003, Uncoupling, BEC 2020.2, MitoFit 2021 BCA, PLoSONE2022ace-sce