Hashimoto 2006 Am J Physiol Endocrinol Metab
| Hashimoto T, Hussien R, Brooks GA (2006) Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol Endocrinol Metab 290:E1237-44. https://doi.org/10.1152/ajpendo.00594.2005 |
Hashimoto T, Hussien R, Brooks GA (2006) Am J Physiol Endocrinol Metab
Abstract: Results of previous studies suggested a role of mitochondria in intracellular and cell-cell lactate shuttles. Therefore, by using a rat-derived L6 skeletal muscle cell line and confocal laser-scanning microscopy (CLSM), we examined the cellular locations of mitochondria, lactate dehydrogenase (LDH), the lactate-pyruvate transporter MCT1, and CD147, a purported chaperone protein for MCT1. CLSM showed that LDH, MCT1, and CD147 are colocalized with the mitochondrial reticulum. Western blots showed that cytochrome oxidase (COX), NADH dehydrogenase, LDH, MCT1, and CD147 are abundant in mitochondrial fractions of L6 cells. Interactions among COX, MCT1, and CD147 in mitochondria were confirmed by immunoblotting after immunoprecipitation. These findings support the presence of a mitochondrial lactate oxidation complex associated with the COX end of the electron transport chain that might explain the oxidative catabolism of lactate and, hence, mechanism of the intracellular lactate shuttle.
• Bioblast editor: Gnaiger E
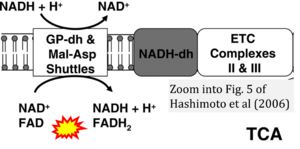
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«