Bektas 2019 Aging (Albany NY)
| Bektas A, Schurman SH, Gonzalez-Freire M, Dunn CA, Singh AK, Macian F, Cuervo AM, Sen R, Ferrucci L (2019) Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy. Aging (Albany NY) 11:9234-63. https://doi.org/10.18632/aging.102438 |
Bektas A, Schurman SH, Gonzalez-Freire M, Dunn CA, Singh AK, Macian F, Cuervo AM, Sen R, Ferrucci L (2019) Aging (Albany NY)
Abstract: To gain understanding on the mechanisms that drive immunosenescence in humans, we examined CD4+ T cells obtained from younger (20-39 years-old) and older (70+ years-old) healthy participants of the Baltimore Longitudinal Study on Aging (BLSA). We found that mitochondrial proteins involved in the electron transport chain were overrepresented in cells from older participants, with prevalent dysregulation of oxidative phosphorylation and energy metabolism molecular pathways. Surprisingly, gene transcripts coding for mitochondrial proteins pertaining to oxidative phosphorylation and electron transport chain pathways were underrepresented in older individuals. Paralleling the observed decrease in gene expression, mitochondrial respiration was impaired in CD4+ T cells from older subjects. Though mitochondrial number in both naïve and memory cells visualized with electron microcopy was similar in older versus younger participants, there were a significantly higher number of autophagosomes, many of them containing undegraded mitochondria, in older individuals. The presence of mitochondria inside the accumulated autophagic compartments in CD4+ T cells from older individuals was confirmed by immunofluorescence. These findings suggest that older age is associated with persistence of dysfunctional mitochondria in CD4+ T lymphocytes caused by defective mitochondrial turnover by autophagy, which may trigger chronic inflammation and contribute to the impairment of immune defense in older persons.
• Bioblast editor: Gnaiger E • O2k-Network Lab: US MD Baltimore Ferrucci L
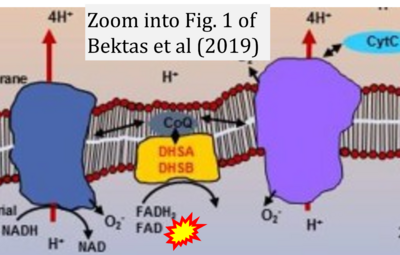
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: MiParea: Respiration, mt-Biogenesis;mt-density Pathology: Aging;senescence
Organism: Human Tissue;cell: Blood cells, Lymphocyte
Coupling state: LEAK, ROUTINE, ET Pathway: ROX HRR: Oxygraph-2k
PBMC