Description
Amplex® UltraRed (AmR) is used as an extrinsic fluorophore for measurement of hydrogen peroxide production (ROS) by cells or mitochondrial preparations. The reaction of H2O2 and AmR is catalyzed by horseradish peroxidase to produce the red fluorescent compound resorufin (excitation wavelength 563 nm, emission 587 nm; the fluorescent product according to the supplier is called UltroxRed in the case of Amplex® UltraRed which has a similar structure to resorufin). The change of emitted fluorescence intensity is directly proportional to the concentration of H2O2 added, whereby the H2O2 is consumed.
Abbreviation: AmR
Reference: Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. https://doi.org/10.26124/bec:2021-0004
MitoPedia methods: Fluorometry
Amplex Red or Amplex UltraRed in research articles
| Citation | Amplex | HRP | pH | Limit of detection | |||
| stock | final | unit definition | stock | final | |||
mM
|
µM
|
U/ml
|
U/ml
|
||||
| BIOTEK | 10
|
50
|
pyrogallol
|
10
|
0.1
|
7.4
|
4 nM (absorption 300 nm)
|
| Life Technologies | 10
|
50
|
10
|
0.1
|
6 to 7.5
|
<80 nM
| |
| Towne 2004 | 160
|
0.41
|
7.5 to 8.5
|
100 nM
| |||
| Zhou1997 | 3 ?
|
0.3 to 1
|
50 nM (10 nM optimal)
| ||||
| Mohanty 1997 | 100
|
10 to 100
|
1
|
18 nM
| |||
| Komary 2010 | 1
|
2.5
|
|||||
Amplex® UltraRed in O2k-FluoRespirometry
O2k-Fluo Smart-Module and O2k-Fluo LED2-Module
- In high-resolution respirometry HRR the Amplex® UltraRed method (Mohanty et al 1997) is used with the O2k-FluoRespirometer selecting the Fluorescence-Sensor Green (O2k-Series D-G: O2k-Fluo LED2-Module) and Smart Fluo-Sensor for O2k-Series H - I. Use black stoppers with black cover-slips to exclude disturbances by external light sources. The fluorescence sensors are inserted though the windows of the O2k-Chambers (Hickey et al 2012). The O2k has also been coupled to a fluorescence spectrophotometer with a light guide inserted through the black PEEK stopper (Anderson 2011).
- The use of AmR in HRR to simultaneously determine respiration and H2O2 flux has been intensively tested in the Oroboros lab (see references).
- AmR reacts with H2O2 catalysed by horseradish peroxidase.
Application in HRR
Amplex® UltraRed
- AmR: Amplex® UltraRed from Life Technologies (former Invitrogen): A36006; 5* 1 mg commercial vials, store at -20°C.
- Preparation of 10 mM stock solution (dissolved in DMSO) - see also Manual Amplex® UltraRed by Life Technologies.
- Storage solution = stock solution
- Prepare a 10 mM storage solution of Amplex® UltraRed reagent (AmR), which will also serve as a stock solution, by adding 340 μL of fresh, high‑quality DMSO (Sigma D8418) to one commercial vial of Amplex® UltraRed reagent (1 mg). Vortex well to dissolve. Protect AmR from light and moisture. The storage solution should then be divided into 20 µL aliquots and stored in the dark in a desiccator, protected from moisture at –20°C for future use. When stored properly, the 10 mM storage solution is stable for 6 months. We have observed that after long-term storage AmR may exert a profound inhibitory effect on cell respiration.
- Storage solution = stock solution
- Note: The preparation of the 10 mM storage solution follows the procedure suggested by the manufacturer. The manufacturer states only an approximate molecular weight for the Amplex® UltraRed formulation and does not publish details how the Amplex® UltraRed formulation deviates from the substance 10-acetyl-3,7-dihydroxyphenoxazine (CAS# 119171-73-2), known as Amplex® Red.
- » O2k manual titrations MiPNet09.12 O2k-Titrations
- Titration into 2 mL O2k-chamber: 2 µL of 10 mM AmR stock solution, final concentration of 10 µM AmR.
- AmR, similar to other chemical probes, may exert an inhibitory effect on mitochondrial and cell respiration. AmR, therefore, should be used at the lowest concentration compatible with the total H2O2 production in an experimental run, including calibrations and chemical background conversion of AmR (Makrecka-Kuka et al 2015; Komlodi et al 2018).
- You may add 1 µL of the 10 mM stock solution for brief measurements to obtain a final concentration of 5 µM. Optimize the final AmR concentration according to respiration media, sample type and concentration, and experimental protocol. These determine the total AmR consumption by H2O2 production during the experiment (check by initial and final H2O2 titrations for calibration). The final concentration of AmR becomes diminished during an experiment due to AmR consumption and dilution by titrations in a SUIT protocol. Check for any potential effects of the experimental AmR concentration on respiratory activity of cells or mt-preparations, by titration of AmR after a specific respiratory state has been reached in a control experiment.
- » O2k manual titrations MiPNet09.12 O2k-Titrations
Color of AmR and the medium
- Amplex UltraRed freshly dissolved should not present any color. The appearance of pink coloring may indicate that the chemical has been compromised.
- During the experimental run, the product UltroxRed (or resorufin), generated from the Amplex UltraRed (or Amplex Red) plus H2O2 in the reaction catalyzed by horseradish peroxidase, accumulates in the chamber. As this chemical has a pink color, it is normal for the medium to have a pink color at the end of the experiment.
Horseradish peroxidase
- Titration into into 2 mL O2k-chamber: 4 µL of HRP stock solution, final concentration 1 U/mL.
- Horse radish peroxidase (Sigma-Aldrich P 8250 - 5 KU): Prepare a stock solution with 500 U HRP/mL in MiR05 or MiR05Cr; the solution can be used as storage solution at -20 °C.
Superoxide dismutase
- Superoxide dismutase (SOD) is included to generate H2O2 from superoxide.
- (Sigma-Aldrich S8160-15KU): We recommend to use the enzyme at 5 U/mL, the final volume to be added to the respiratory chamber has thus to be adjusted accordingly.
- Experiments can be performed in the absence of SOD.
DTPA
- » See: DTPA
Calibration with H2O2
- Calibration standards of H2O2: Commercial solution (Sigma-Aldrich 323381 - 25 mL hydrogen peroxide solution 3 wt.%; stabilized with acetanilide, c. 200 ppm) = 880 mM H2O2.
- Prepare a HCl stock solution of 10 µM HCl.
- H2O2 dilution 1 (1:88): add 10 µL of a commercial stock solution of 3 wt. H2O2 to 870 µL 10 µM HCl solution, to obtain a 10 mM H2O2 solution.
- H2O2 dilution 2 (1:250):add 4 µL of 10 mM H2O2 solution to 996 µL 10 µM HCl solution, to obtain the H2O2 stock solution of 40 µM.
- The H2O2 calibration solution has to be prepared fresh every day, kept away from light exposure, and freezing should be avoided.
- Calibration standards of H2O2: Commercial solution (Sigma-Aldrich 323381 - 25 mL hydrogen peroxide solution 3 wt.%; stabilized with acetanilide, c. 200 ppm) = 880 mM H2O2.
- Titration into 2 mL O2k-chamber: 5 µL of the 40 µM H2O2 stock, step increase of 0.1 µM H2O2.
- Background H2O2 calibration:
- A set of H2O2 titrations conducted after the addition of respiration medium, (DTPA), HRP and Amplex® UltraRed to the O2k-chamber but before sample addition.
- This step is required (1) for the calculation of chemical background fluorescence; see: MiPNet24.10 H2O2 flux analysis and How to analyze H2O2 flux in DatLab, and (2) for calculation of the sensitivity of the Amplex® UltraRed assay towards H2O2, which, therefore, serves as a quality control step. The sensitivity depends on the respiration medium and on the components of the Amplex® UltraRed assay, and in the same respiration medium, the sensitivity of the AmR assay should be in the same range. For more information: Komlodi 2018 Methods Mol Biol.
- Background H2O2 calibration has to be performed for each individual instrumental setting (O2k, fluorescence sensor and chamber), if a new stock solution of DTPA, HRP, SOD, Amplex® UltraRed, or H2O2, is prepared, or a different respiration medium is used. As the H2O2 stock solution needs to be freshly prepared, it is recommended to perform a background H2O2 calibration before each experiment and at a minimum once per experimental day.
- The protocol for background H2O2 calibration is provided with DatLab7.4. For further information, see: Instrumental: Browse DL-Protocols and templates
- Afterwards we suggest to perform calibrations at the beginning (sample already present), intermittently at various respiratory states, and near the end of an experiment. These calibrations steps are recommended because over the experimental time the sensitivity decreases and chemicals can influence the sensitivity of the AmR assay towards H2O2. After the addition of the biological sample, the sensitivity usually decreases owing to the (high) antioxidant capacity of the sample. For further information, see: Komlodi 2018 Methods Mol Biol
- Why it is necessary to perform multiple H2O2 calibrations in the Amplex® UltraRed (AmR) assay?
- The sensitivity to H2O2 of the Amplex® UltraRed assay changes during the experiment, thus requires multiple calibrations. These sensitivity changes may be the function of (1) experimental time and accumulating UltroxRed (similar to resorufin), (2) changes of the optical properties due to titrations, (3) the radical scavenging capacity of the sample. Therefore, H2O2 calibrations are performed before and after sample addition, and after selected titration steps. - »Komlodi 2018 Methods Mol Biol«
- The DLPs for AmR SUIT protocols already come with multiple steps of H2O2 calibrations implemented.- » SUITbrowser«
- DatLab supports automatic H2O2 calibration by calculating the calibration parameters by linear regression and graphical display of the calibration regression.
- Problem: Here is a DatLab file where I ran the H2O2 calibration protocol with 6 H2O2 titrations. I drew up 5 uL of H2O2 into the syringe for titration into each chamber individually for the first two titrations. For the remaining titrations, I drew up 10 uL of H2O2 into the syringe and added 5 uL to one chamber and the rest into the second chamber. For titrations 3 and 4 I added to chamber B first, followed by chamber A. For titrations 5 and 6 I added to chamber A first, followed by chamber B. If sensitivities are measured for each set of titrations based on the manner in which H2O2 was added to the chambers, it can be seen that the chamber which is titrated second when 10 µL of H2O2 is drawn into the syringe at one time has reduced sensitivity measurements. When fresh H2O2 is drawn up for each chamber individually, the sensitivities between chambers are much more comparable. (The numbers shown in the figures are sensitivities expressed as V/µM.)
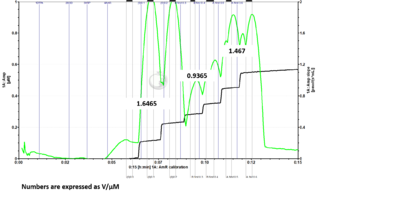
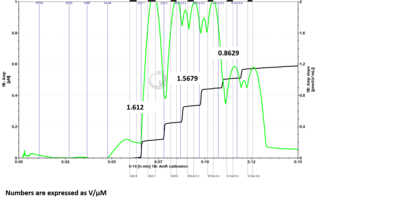
- Answer: It is recommended to fill the syringe with H2O2 each time with 5 µL for each O2k-Chamber instead of filling up the syringe with 10 µL and titrate one chamber after the other. Based on tests of Jennifer Norman (US_CA Davis_Roshanravan B) it seems that either the syringe for H2O2 titration is not precise filling it up with the maximal volume or H2O2 might be degraded by light already in the syringe.
Experimental media for the AmR assay
- Media with high antioxidant activity compete with HRP and partially consume H2O2 before it can react with AmR to form the active fluorophore UltroxRed (similar to resorufin). This was shown by comparing the resorufin-sensitivity and H2O2-sensitivity (Krumschnabel et al 2015; Komlodi et al 2018).
- H2O2-sensitivity: the change of fluorescence signal per µM H2O2 added in calibrations with H2O2 titration in media containing HRP and AmR.
- Resorufin-sensitivity: the change of fluorescence signal per µM resorufin added in calibrations with resorufin titration in media containing HRP and AmR.
- Media with high antioxidant activity compete with HRP and partially consume H2O2 before it can react with AmR to form the active fluorophore UltroxRed (similar to resorufin). This was shown by comparing the resorufin-sensitivity and H2O2-sensitivity (Krumschnabel et al 2015; Komlodi et al 2018).
- The H2O2-sensitivity is much higher in a simple phosphate buffer compared to media with strong antioxidant capacity. In contrast, this is not the case for the resorufin-sensitivity.
H2O2 sensitivity in MiR05
- We recommend running experiments in MiR05 with a sensitivity >0.5 and <3 at gain 1000 and fluorescence intensity lower than 500 . If the sensitivity is out of this range, please change the light intensity of the Fluo-sensors (in DatLab 7.4: [Oroboros O2k] \ [O2k control], click on the tab [Amperometric, Amp] and change light intensity (Amp polarization voltage [mV]). Do the calibration again by titrating H2O2 to check the sensitivity.
- The sensitivity values may vary depending on the batch of medium and chemicals used.
- The sensitivity value is used to calibrate the amperometric raw signal [V] to H2O2 equivalent concentration [µM]. If experiments present different sensitivities, the resulting H2O2 fluxes after calibration can still be comparable, as long as the sensitivity values are in the recommended range and the same settings are used for all experiments.
Calculation of background fluorescence slope of MiR05
- In the absence of sample, there is a spontaneous increase of the Ultroxred fluorescence signal over time, the extent of which depends on components of the respiration medium (Krumschnabel et al 2015). The sensitivity – the change in fluorescence per unit of H2O2 produced in or added to the chamber – depends on the medium and tends to decline over time. Both the background change in fluorescence and the change in assay sensitivity over time need to be corrected for in data analysis, for which DatLab-Analysis templates are available.
- The Excel template is ideal for analysis of O2 and H2O2 fluxes measured in MiR05-Kit supplemented with DTPA. The background fluorescence slope of the AmR assay is dependent on the Lot number of the MiR05-Kit, therefore, in each SUIT-###_AmR folder, in the Excel templates for MiR05-Kit (supplemented with DTPA) you can find different analysis templates for each Lot number. The Excel sheets differ from each other in the correction for the background fluorescence slope. You need to use the Excel template which corresponds with the Lot# of MiR05-Kit you use. If you do not add DTPA or use homemade MiR05 instead of the MiR05-Kit, we recommend calculating the background fluorescence slope. The calculation of the background fluorescence slope is also advisable when a new batch of Amplex UltraRed is used or a newly prepared MiR05 is applied. Please follow the steps to measure and calculate background fluorescence slope:
- The chemical background flux can be measured as follows: File:AmR background fluorescence slope.DLP
- In DatLab 7.4, set the marks to the Amp slope according to the protocol.
- a. Go to [Layout] menu and click into ‘O2&Amp´ and select `Standard Layouts/01 Amp Amperometric_Raw signal´.
- b. Go to `Marks´ and select ‘Slope uncorrected + all info´. In the new window select `AmR slope [mV/s]´ in `Plot for Marks´.
- c. Channel: `Amperometric,Amp´. Leave only this channel selected.
- d. Select: `Median´.
- e. Sort by: `Time´(default).
- f. Then, click on [Copy to clipboard] to copy the selected values.
- In the Amp background fluorescence slope.xlsx Excel template (see below): Click on the yellow cell A1 and paste [Ctrl+V] Amp slope from DatLab. If more than one experiment is performed, copy the Amp slope on A32, A63, A94, A126 or A158.
- In the "Data" sheet, write the number of the FluoSensors in cells B12. If more than one sensor was tested, write the sensor number in cells B43, B74, B105, B137 or B169. The numbers can be found in the O2k control window (select in ´Oroboros O2k´ menu or press F7), ´Amperometric, Amp´ tab.
- Equation required for the correction for the background fluorescence slope using the applied FluoSensor can be found in the figure. The values can be also found in the cells H18 and I18, H48 and I48, H79 and I79, H110 and I110, H142 and I42 or H174 and I174.
- It is advisable to measure thebackground fluorescence slope with the same FluoSensors in the same O2k-chambers several times and then calculate the plot of the equation from more experiments. In the Excel template in the ´Summary and equation´ tab, you can copy a° and b° into the table to calculate the equation of plot.
- In the SUIT-###_AmR analysis templates, modify the values in the equation for the background fluorescence slope (cells N66, W66, and X66).
- The Excel templates are available here: File:AmR background fluorescence slope.xlsx File:AmR background fluorescence slope demo.xlsx
- In the absence of sample, there is a spontaneous increase of the Ultroxred fluorescence signal over time, the extent of which depends on components of the respiration medium (Krumschnabel et al 2015). The sensitivity – the change in fluorescence per unit of H2O2 produced in or added to the chamber – depends on the medium and tends to decline over time. Both the background change in fluorescence and the change in assay sensitivity over time need to be corrected for in data analysis, for which DatLab-Analysis templates are available.
HRP-independent background AmR flux
- A study by Miwa et al 2016 (Free Radical Bio Med 90:173) suggests that the HRP-independent artificial background is related to a mitochondrially expressed carboxylesterase which converts Amplex® Red to resorufin at a high rate. The issue can be relatively easily solved by adding a protease inhibitor to various mitochondrial preparations as described here.
- For further information see the Discussion page.
AmR assay dilution
- How to minimize dilution of the AmR assay upon sample addition
- To minimize the dilution of the AmR assay either:
- 1.) Decrease the titration volume of the sample by using a more concentrated sample solution
- 2.) Titrate additionally the components of the AmR assay together with the sample
- 3.) After AmR background calibration, empty and wash the chamber, then add buffer and sample and start titrating the components of the AmR assay into the closed O2k-chamber.
- In either case 2 or 3, use media and chemicals from the same batch.
- Of note, performing H2O2 calibration directly after sample addition allows for calculating the sensitivity to correct the fluorescence signal for sample dilution.
- To minimize the dilution of the AmR assay either:
- How to minimize dilution of the AmR assay upon sample addition
Artefacts
Liver homogenate
- Liver homogenate cannot be used with the Amplex® UltraRed assay. - See:Tissue homogenate
Injection artefact
NADH and reduced glutathione
- Votyakova TV, Reynolds IJ (2014 Arch Biochem Biophys 431:138-44) showed that NADH and reduced glutathione are able to react with AmR in the presence of O2 and HRP and create a background in the absence of mitochondria. If the mitochondria remain intact, this above-mentioned reaction is negligible. If you add SOD (superoxide dismutase), you can avoid this side-effect of the AmR assay.
Substances incompatible with the Amplex® UltraRed assay
- The following substances/ classes of substances are strictly incompatible with the Amplex® Red method for theoretical reasons:
- Strongly redox-active substances, e.g. cytochrome c, TMPD/Ascorbate
- Inhibitors of horseradish peroxidase, e.g. cyanide, azide; more information: horseradish peroxidase
- Catalase and other substances consuming or scavenging H2O2. The effect of substances in the medium that slowly consume H2O2 is taken into account by the calibration procedure. However, such substances decrease the sensitivity of the method. Note that catalase can be a valuable tool for checking artefacts. See:Avoiding artefacts.
- The effect of other substances should be checked by experiments without biological sample, including comparing the sensitivity (result of calibration) before and after injecting the substances.
- In preliminary experiments Oroboros Instruments evaluated small amounts (as typically used in SUIT protocols) of the following substances as compatible with the method:
- DMSO, ethanol, malate, glutamate, pyruvate (a strong scavanger of H2O2), succinate, (ADP + Mg2+), (ATP + Mg2+), rotenone, FCCP, CCCP, oligomycin, antimycin A, malonate, myxothiazol
Avoiding artefacts
- The Amplex® method is based on the H2O2 dependent oxidation of AmR to UltroxRed by HRP. Under unfavorable conditions AmR may be oxidized even in the absence of H2O2. At a small rate such an oxidation occurs in the presence of HRP even without any sample present. The magnitude of this background signal ("drift") depends, among other things, on the light intensity used and can therefore be minimized by using the suggested or lower light intensity. Components of the sample may however induce a far higher, non H2O2 related rate of AmR oxidation. Therefore, especially when applying the method on new types of samples the method should be checked for artifacts. A few approaches are listed here:
- Sequential addition of HRP and AmR: This method is particular easy to implement if AmR and HRP have to be added to the chamber already containing the sample anyway: First, inject Amplex® Red and wait a few minutes for flux stabilization. The Amp slope has to stay near zero. Then add HRP. The Amp slope should increase and correspond to the H2O2 production. If a significant Amp slope is detected before the addition of HRP this increase in fluorescence is not caused by H2O2 production. The experiment can be continued as usual after this test. If the sample is injected routinely into the chamber already containing AmR and HRP the method can not be applied. In this case, it is suggested to change this sequence at least for one experiment.
- Addition of catalase: After a H2O2 flux (production) is established, a high dose (e.g. 10 µL of a 280,000 U/mL stock solution) of catalase is injected. Catalase competes with HRP for the available H2O2. Then the apparent H2O2 flux (the Amp slope) should be reduced to near zero as a control for distinguishing an unspecific chemical background slope from H2O2 dependent Amplex® UltraRed oxidation.
- Note: The experiment cannot be continued afterwards for measurement of hydrogen peroxide production, whereas respiration can be recorded onwards.
Oxygen range
- The H2O2 production of mitochondria is oxygen dependent Komlodi 2021 BEC AmR-O2. The oxygen concentration can be controlled in the O2k-chamber while running the AmR assay. We recommend running the assay at low O2 concentration, e.g., between 60 and 30 µM O2. If this is not possible, it is advisable to run all assays in the same [O2] range, and take marks for different conditions under a similar [O2] to minimize variability.
Nitrogen or hydrogen injection
- To decrease the oxygen concentration in the O2k-chamber using nitrogen or hydrogen see Setting the oxygen concentration.
- Check Oxia for fast and safe production of hydrogen (and oxygen).
Permeabilized fibers
- Why are permeabilized muscle fibers (pfi) a poor sample type for studying reactive oxygen species (ROS) production?
- In experiments with pfi, high oxygen concentrations are needed to avoid oxygen limitation. However, the high oxygen pressures used may artificially increase ROS, including H2O2 production, making pfi a less optimal model for ROS production studies. See: Oxygen dependence of pfi
Experimental SOP
H2O2 flux analysis and mark setting
- Amp slope smoothing in DatLab
- MiPNet24.10 H2O2 flux analysis
- H2O2 flux analysis video
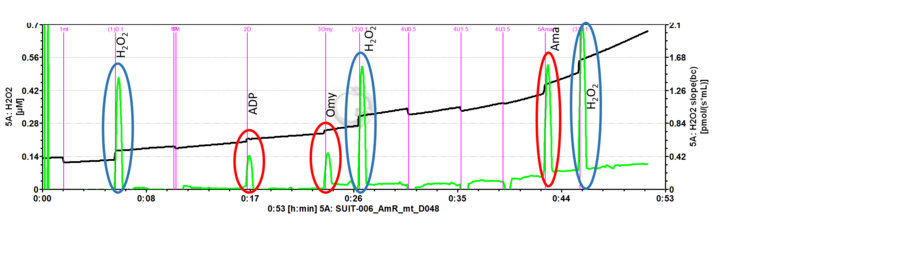
- Mark setting: The black line refers to the raw fluorescence signal [V] of the product of the AmR assay called resorufin (UltroxRed in the case of Amplex® UltraRed), while the green line is the fluorescence slope [mV/s] automatically calculated from the black line.
Excel analysis templates
- Excel templates are provided for analysis of the H2O2 measurements. It can be found in the upper menu in DatLab in Protocols/SUIT: Browse DL-Protocols and templates and then select your protocol (e.g. SUIT-009/SUIT-009_AmR/SUIT-009_AmR_ce-pce_D019).
- The calculations used in the excel analysis template are provided complying with Oroboros transparency policy: [1]
- The excel analysis templates are updated for the respective SUIT protocol. The latest versions are available here (last update 2021-09-20):
- For SUIT-006 AmR mt D048 protocol, see: File:SUIT-006 AmR mt D048 excel.xlsx and File:SUIT-006 AmR mt D048 demo.xlsx
- For SUIT-009 AmR ce-pce D019 protocol, see: File:SUIT-009 AmR ce-pce D019 excel.xlsx and File:SUIT-009 AmR ce-pce D019 demo.xlsx
- For SUIT-013 AmR ce D023 protocol, see: File:SUIT-013 AmR ce D023 excel.xlsx and File:SUIT-013 AmR ce D023 demo.xlsx
- For SUIT-018 AmR mt D031 protocol, see: File:SUIT-018 AmR mt D031 excel.xlsx and File:SUIT-018 AmR mt D031 demo.xlsx
- For SUIT-018 AmR mt D041 protocol, see: File:SUIT-018 AmR mt D041 excel.xlsx and File:SUIT-018 AmR mt D041 demo.xlsx
- For SUIT-026 AmR mt D064 protocol, see: File:SUIT-026 AmR mt D064 excel.xlsx and File:SUIT-026 AmR mt D064 demo.xlsx
- Manual: MiPNet24.10 H2O2 flux analysis
SUIT protocols
- SUIT-006 AmR mt D048: to study the influence of mt-membrane potential on NADH-linked H2O2 flux
- SUIT-013 AmR ce D023: to detect H2O22 flux in living cells
- SUIT-018: to detect NS-linked H2O2 flux at different O2 concentrations
- SUIT-026 AmR mt D064: to detect RET-linked H2O2 flux
Technical support
Set marks to calibrate the fluorescence signal using Amplex® UltraRed assay
- To calibrate the fluorescence signal in molar units [µM], set the marks on the black plot.
Set marks to analyse H2O2 flux
Data analysis with the Excel template
Defective fluorescence module
Exchange of filters in the fluorescence sensors
How to connect the Smart Fluo-Sensors to the O2k?
References
- Mohanty JG, Jaffe JS, Schulman ES, Raible DG (1997) A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. https://doi.org/10.1016/S0022-1759(96)00244-X - »Bioblast link«
- Hickey AJ, Renshaw GM, Speers-Roesch B, Richards JG, Wang Y, Farrell AP, Brauner CJ (2012) A radical approach to beating hypoxia: depressed free radical release from heart fibers of the hypoxia-tolerant epaulette shark (Hemiscyllum ocellatum). https://doi.org/10.1007/s00360-011-0599-6 - »Bioblast link«
- Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR, Kypson AP (2011) Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. https://doi.org/10.1152/ajpheart.00932.2010 - »Bioblast link«
- Makrecka-Kuka M, Krumschnabel G, Gnaiger E (2015) High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. https://doi.org/10.3390/biom5031319 - »Bioblast link«
- Komlodi T, Sobotka O, Krumschnabel G, Bezuidenhout N, Hiller E, Doerrier C, Gnaiger E (2018) Comparison of mitochondrial incubation media for measurement of respiration and hydrogen peroxide production. https://doi.org/10.1007/978-1-4939-7831-1_8 - »Bioblast link«
- Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, Heidler J, Gauper K, Fasching M, Gnaiger E (2015) Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. https://doi.org/10.1007/978-1-4939-2257-4_22 - »Bioblast link«
- Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. https://doi.org/10.26124/bec:2021-0004
- Komary Z, Tretter L, Adam-Vizi V (2010) Membrane potential-related effect of calcium on reactive oxygen species generation in isolated brain mitochondria. https://doi.org/10.1016/j.bbabio.2010.03.010 - Komary 2010 Biochim Biophys Acta
- Mishin V, Gray JP, Heck DE, Laskin DL, Laskin JD (2010) Application of Amplex red/horseradish peroxidase assay to measure hydrogen peroxide production by recombinant microsomal enzymes. https://doi.org/10.1016/j.freeradbiomed.2010.02.030 - Mishin 2010 Free Radical Biol Med
- Towne V, Will M, Oswald B, Zhao Q (2004) Complexities in horseradish peroxidase-catalyzed oxidation of dihydroxyphenoxazine derivatives: appropriate ranges for pH values and hydrogen peroxide concentrations in quantitative analysis. https://doi.org/10.1016/j.ab.2004.07.037 - Towne 2004 Anal Biochem
- Tretter Laszlo, Adam-Vizi Vera (2012) High Ca2+ load promotes hydrogen peroxide generation via activation of α-glycerophosphate dehydrogenase in brain mitochondria. https://doi.org/10.1016/j.freeradbiomed.2012.09.029 - Tretter 2012 Free Radic Biol Med
- Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP (1997) A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. https://doi.org/10.1006/abio.1997.2391 - Zhou 1997 Anal Biochem
O2k-Publications: H2O2
- List of publications: Amplex UltraRed - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
| Year | Reference | Organism | Tissue;cell | Stress | Diseases | |
|---|---|---|---|---|---|---|
| Al-Sabri 2024 Sci Rep | 2024 | Al-Sabri MH, Ammar N, Korzh S, Alsehli AM, Hosseini K, Fredriksson R, Mwinyi J, Williams MJ, Boukhatmi H, Schiöth HB (2024) Fluvastatin-induced myofibrillar damage is associated with elevated ROS, and impaired fatty acid oxidation, and is preceded by mitochondrial morphological changes. https://doi.org/10.1038/s41598-024-53446-w | Drosophila | Skeletal muscle | ||
| Cefis 2024 Acta Physiol (Oxf) | 2024 | Cefis M, Dargegen M, Marcangeli V, Taherkhani S, Dulac M, Leduc-Gaudet JP, Mayaki D, Hussain SNA, Gouspillou G (2024) MFN2 overexpression in skeletal muscles of young and old mice causes a mild hypertrophy without altering mitochondrial respiration and H2O2 emission. Acta Physiol (Oxf) [Epub ahead of print]. https://doi.org/10.1111/apha.14119 | Mouse | Skeletal muscle | Aging;senescence | |
| Czyzowska 2023 Redox Biol | 2023 | Czyżowska A, Brown J, Xu H, Sataranatarajan K, Kinter M, Tyrell VJ, O'Donnell VB, Van Remmen H (2023) Elevated phospholipid hydroperoxide glutathione peroxidase (GPX4) expression modulates oxylipin formation and inhibits age-related skeletal muscle atrophy and weakness. https://doi.org/10.1016/j.redox.2023.102761 | Mouse | Skeletal muscle | Aging;senescence | |
| Dominguez-Lopez 2023 Neuropharmacology | 2023 | Dominguez-Lopez S, Ahn B, Sataranatarajan K, Ranjit R, Premkumar P, Van Remmen H, Beckstead MJ (2023) Long-term methamphetamine self-administration increases mesolimbic mitochondrial oxygen consumption and decreases striatal glutathione. https://doi.org/10.1016/j.neuropharm.2023.109436 | Mouse | Nervous system | ||
| Fletcher 2023 Transl Res | 2023 | Fletcher E, Miserlis D, Sorokolet K, Wilburn D, Bradley C, Papoutsi E, Wilkinson T, Ring A, Ferrer L, Haynatzki G, Smith RS, Bohannon WT, Koutakis P (2023) Diet-induced obesity augments ischemic myopathy and functional decline in a murine model of peripheral artery disease. https://doi.org/10.1016/j.trsl.2023.05.002 | Mouse | Skeletal muscle | Myopathy Obesity | |
| Gautam 2023 Neurobiol Dis | 2023 | Gautam M, Genç B, Helmold B, Ahrens A, Kuka J, Makrecka-Kuka M, Günay A, Koçak N, Aguilar-Wickings IR, Keefe D, Zheng G, Swaminathan S, Redmon M, Zariwala HA, Özdinler PH (2023) SBT-272 improves TDP-43 pathology in ALS upper motor neurons by modulating mitochondrial integrity, motility, and function. https://doi.org/10.1016/j.nbd.2023.106022 | Rat | Heart Nervous system | Neurodegenerative | |
| Steffen 2023 J Exp Biol | 2023 | Steffen JBM, Sokolov EP, Bock C, Sokolova IM (2023) Combined effects of salinity and intermittent hypoxia on mitochondrial capacity and reactive oxygen species efflux in the Pacific oyster, Crassostrea gigas. https://doi.org/10.1242/jeb.246164 | Molluscs | Lung;gill | Oxidative stress;RONS Hypoxia | |
| Som 2023 Am J Physiol Cell Physiol | 2023 | Som R, Fink BD, Yu L, Sivitz WI (2023) Oxaloacetate regulates complex II respiration in brown fat: dependence on UCP1 expression. Am J Physiol Cell Physiol 324:C1236-48. doi: 10.1152/ajpcell.00565.2022 | Mouse | Fat | Oxidative stress;RONS | Obesity |
| Leduc-Gaudet 2023 Nat Commun | 2023 | Leduc-Gaudet JP, Franco-Romero A, Cefis M, Moamer A, Broering FE, Milan G, Sartori R, Chaffer TJ, Dulac M, Marcangeli V, Mayaki D, Huck L, Shams A, Morais JA, Duchesne E, Lochmuller H, Sandri M, Hussain SNA, Gouspillou G (2023) MYTHO is a novel regulator of skeletal muscle autophagy and integrity. https://doi.org/10.1038/s41467-023-36817-1 | Mouse | Skeletal muscle | ||
| Pharaoh 2023 Geroscience | 2023 | Pharaoh G, Kamat V, Kannan S, Stuppard RS, Whitson J, Martín-Pérez M, Qian WJ, MacCoss MJ, Villén J, Rabinovitch P, Campbell MD, Sweet IR, Marcinek DJ (2023) The mitochondrially targeted peptide elamipretide (SS-31) improves ADP sensitivity in aged mitochondria by increasing uptake through the adenine nucleotide translocator (ANT). https://doi.org/10.1007/s11357-023-00861-y | Mouse | Skeletal muscle | Aging;senescence | |
| Salmon 2023 Geroscience | 2023 | Salmón P, Millet C, Selman C, Monaghan P, Dawson NJ (2023) Tissue-specific reductions in mitochondrial efficiency and increased ROS release rates during ageing in zebra finches, Taeniopygia guttata. https://doi.org/10.1007/s11357-022-00624-1 | Birds | Skeletal muscle Liver | Oxidative stress;RONS | Aging;senescence |
| Batterson 2023 Physiol Rep | 2023 | Batterson PM, McGowan EM, Borowik AK, Kinter MT, Miller BF, Newsom SA, Robinson MM (2023) High-fat diet increases electron transfer flavoprotein synthesis and lipid respiration in skeletal muscle during exercise training in female mice. https://doi.org/10.14814/phy2.15840 | Mouse | Skeletal muscle | ||
| Devaux 2023 J Comp Physiol B | 2023 | Devaux JBL, Hedges CP, Birch N, Herbert N, Renshaw GMC, Hickey AJR (2023) Electron transfer and ROS production in brain mitochondria of intertidal and subtidal triplefin fish (Tripterygiidae). https://doi.org/10.1007/s00360-023-01495-4 | Fishes | Nervous system | Oxidative stress;RONS | |
| Stouth 2023 Autophagy | 2023 | Stouth DW, vanLieshout TL, Mikhail AI, Ng SY, Raziee R, Edgett BA, Vasam G, Webb EK, Gilotra KS, Markou M, Pineda HC, Bettencourt-Mora BG, Noor H, Moll Z, Bittner ME, Gurd BJ, Menzies KJ, Ljubicic V (2023) CARM1 drives mitophagy and autophagy flux during fasting-induced skeletal muscle atrophy. https://doi.org/10.1080/15548627.2023.2288528 | Mouse | Skeletal muscle | ||
| Pileggi 2022 EBioMedicine | 2022 | Pileggi CA, Blondin DP, Hooks BG, Parmar G, Alecu I, Patten DA, Cuillerier A, O'Dwyer C, Thrush AB, Fullerton MD, Bennett SAL, Doucet É, Haman F, Cuperlovic-Culf M, McPherson R, Dent RRM, Harper ME (2022) Exercise training enhances muscle mitochondrial metabolism in diet-resistant obesity. https://doi.org/10.1016/j.ebiom.2022.104192 | Human | Skeletal muscle | Obesity | |
| Loughland 2022 J Exp Biol | 2022 | Loughland I, Lau GY, Jolly J, Seebacher F (2022) Rates of warming impact oxidative stress in zebrafish (Danio rerio). | Zebrafish | Skeletal muscle | Oxidative stress;RONS | |
| Xu 2022 Sci Adv | 2022 | Xu H, Ahn B, Van Remmen H (2022) Impact of aging and oxidative stress on specific components of excitation contraction coupling in regulating force generation. https://doi.org/10.1126/sciadv.add7377 | Mouse | Skeletal muscle | Oxidative stress;RONS | Aging;senescence |
| Smith 2022 J Pineal Res | 2022 | Smith KLM, Swiderska A, Lock MC, Graham L, Iswari W, Choudhary T, Thomas D, Kowash HM, Desforges M, Cottrell EC, Trafford AW, Giussani DA, Galli GLJ (2022) Chronic developmental hypoxia alters mitochondrial oxidative capacity and reactive oxygen species production in the fetal rat heart in a sex-dependent manner. https://doi.org/10.1111/jpi.12821 | Rat | Heart | Hypoxia | |
| Yoval-Sanchez 2022 Redox Biol | 2022 | Yoval-Sánchez B, Ansari F, James J, Niatsetskaya Z, Sosunov S, Filipenko P, Tikhonova IG, Ten V, Wittig I, Rafikov R, Galkin A (2022) Redox-dependent loss of flavin by mitochondria complex I is different in brain and heart. https://doi.org/10.1016/j.redox.2022.102258 | Mouse | Heart Nervous system | Ischemia-reperfusion | |
| Brown 2022 Redox Biol | 2022 | Brown JL, Peelor FF 3rd, Georgescu C, Wren JD, Kinter M, Tyrrell VJ, O'Donnell VB, Miller BF, Van Remmen H (2022) Lipid hydroperoxides and oxylipins are mediators of denervation induced muscle atrophy. https://doi.org/10.1016/j.redox.2022.102518 | Mouse | Skeletal muscle | Other | |
| VanLieshout 2022 Mol Metab | 2022 | vanLieshout TL, Stouth DW, Hartel NG, Vasam G, Ng SY, Webb EK, Rebalka IA, Mikhail AI, Graham NA, Menzies KJ, Hawke TJ, Ljubicic V (2022) The CARM1 transcriptome and arginine methylproteome mediate skeletal muscle integrative biology. https://doi.org/10.1016/j.molmet.2022.101555 | Mouse | Skeletal muscle | ||
| Lopes 2022 Int J Mol Sci | 2022 | Lopes JA, Collino F, Rodrigues-Ferreira C, Sampaio LDS, Costa-Sarmento G, Wendt CHC, Almeida FP, Miranda KR, Kasai-Brunswick TH, Lindoso RS, Vieyra A (2022) Early effects of extracellular vesicles secreted by adipose tissue mesenchymal cells in renal ischemia followed by reperfusion: mechanisms rely on a decrease in mitochondrial anion superoxide production. https://doi.org/10.3390/ijms23062906 | Human | Kidney | Ischemia-reperfusion | |
| Munro 2022 Mitochondrion | 2022 | Munro D, Rodríguez E, Blier PU (2022) The longest-lived metazoan, Arctica islandica, exhibits high mitochondrial H2O2 removal capacities. https://doi.org/10.1016/j.mito.2022.11.005 | Molluscs | Lung;gill Endothelial;epithelial;mesothelial cell | Oxidative stress;RONS | Aging;senescence |
| McKenna 2022 J Appl Physiol (1985) | 2022 | McKenna CF, Salvador AF, Keeble AR, Khan NA, De Lisio M, Konopka AR, Paluska SA, Burd NA (2022) Muscle strength after resistance training correlates to mediators of muscle mass and mitochondrial respiration in middle-aged adults. https://doi.org/10.1152/japplphysiol.00186.2022 | Human | Skeletal muscle | ||
| Qvit 2022 Pharmaceuticals (Basel) | 2022 | Qvit N, Lin AJ, Elezaby A, Ostberg NP, Campos JC, Ferreira JCB, Mochly-Rosen D (2022) A selective inhibitor of cardiac troponin I phosphorylation by delta protein kinase C (δPKC) as a treatment for ischemia-reperfusion injury. https://doi.org/10.3390/ph15030271 | Rat | Heart | Ischemia-reperfusion | |
| Salyers 2022 JVS Vasc Sci | 2022 | Salyers ZR, Mariani V, Balestrieri N, Kumar RA, Vugman NA, Thome T, Villani KR, Berceli SA, Scali ST, Vasilakos G, Ryan TE (2022) S100A8 and S100A9 are elevated in chronically threatened ischemic limb muscle and induce ischemic mitochondrial pathology in mice. https://doi.org/10.1016/j.jvssci.2022.03.003 | Human Mouse | Skeletal muscle | Cardiovascular | |
| Sarabhai 2022 Diabetologia | 2022 | Sarabhai T, Mastrototaro L, Kahl S, Bönhof GJ, Jonuscheit M, Bobrov P, Katsuyama H, Guthoff R, Wolkersdorfer M, Herder C, Meuth SG, Dreyer S, Roden M (2022) Hyperbaric oxygen rapidly improves tissue-specific insulin sensitivity and mitochondrial capacity in humans with type 2 diabetes: a randomised placebo-controlled crossover trial. https://doi.org/10.1007/s00125-022-05797-0 | Human | Skeletal muscle Fat | Diabetes | |
| Hansen 2022 Free Radic Biol Med | 2022 | Hansen C, Møller S, Ehlers T, Wickham KA, Bangsbo J, Gliemann L, Hellsten Y (2022) Redox balance in human skeletal muscle-derived endothelial cells - Effect of exercise training. https://doi.org/10.1016/j.freeradbiomed.2021.12.265 | Human | Endothelial;epithelial;mesothelial cell | ||
| Belosludtseva 2022 Membranes (Basel) | 2022 | Belosludtseva NV, Pavlik LL, Belosludtsev KN, Saris NL, Shigaeva MI, Mironova GD (2022) The short-term opening of cyclosporin A-independent palmitate/Sr 2+-induced pore can underlie ion efflux in the oscillatory mode of functioning of rat liver mitochondria. https://doi.org/10.3390/membranes12070667 | Rat | Liver | ||
| Starr 2022 Curr Res Physiol | 2022 | Starr VJ, Dzialowski EM (2022) Developing chicken cardiac muscle mitochondria are resistant to variations in incubation oxygen levels. https://doi.org/10.1016/j.crphys.2022.03.001 | Chicken | Heart | Hypoxia | |
| Cruz-Gregorio 2022 Antioxidants (Basel) | 2022 | Cruz-Gregorio A, Aranda-Rivera AK, Aparicio-Trejo OE, Medina-Campos ON, Sciutto E, Fragoso G, Pedraza-Chaverri J (2022) GK-1 Induces oxidative stress, mitochondrial dysfunction, decreased membrane potential, and impaired autophagy flux in a mouse model of breast cancer. https://doi.org/10.3390/antiox12010056 | Mouse | Endothelial;epithelial;mesothelial cell | Cancer | |
| Formiga-Jr 2022 J Vis Exp | 2022 | Formiga-Jr MA, Camacho-Pereira J (2022) Assessing mitochondrial function in sciatic nerve by high-resolution respirometry. https://doi.org/10.3791/63690 | Mouse | Nervous system | ||
| Jasz 2021 J Cell Mol Med | 2021 | Jász DK, Szilágyi ÁL, Tuboly E, Baráth B, Márton AR, Varga P, Varga G, Érces D, Mohácsi Á, Szabó A, Bozó R, Gömöri K, Görbe A, Boros M, Hartmann P (2021) Reduction in hypoxia-reoxygenation-induced myocardial mitochondrial damage with exogenous methane. https://doi.org/10.1111/jcmm.16498 | Rat | Heart | Ischemia-reperfusion | |
| Morgan 2021 Resuscitation | 2021 | Morgan RW, Sutton RM, Himebauch AS, Roberts AL, Landis WP, Lin Y, Starr J, Ranganathan A, Delso N, Mavroudis CD, Volk L, Slovis J, Marquez AM, Nadkarni VM, Hefti M, Berg RA, Kilbaugh TJ (2021) A randomized and blinded trial of inhaled nitric oxide in a piglet model of pediatric cardiopulmonary resuscitation. Resuscitation 162:274-83 . | Pig | Nervous system | Cardiovascular | |
| Marrocco 2021 J Immunol | 2021 | Marrocco A, Frawley K, Pearce LL, Peterson J, O'Brien JP, Mullett SJ, Wendell SG, St Croix CM, Mischler SE, Ortiz LA (2021) Metabolic adaptation of macrophages as mechanism of defense against crystalline silica. J Immunol 207:1627-40. | Mouse | Macrophage-derived | Other | |
| Newsom 2021 Med Sci Sports Exerc | 2021 | Newsom SA, Stierwalt HD, Ehrlicher SE, Robinson MM (2021) Substrate-specific respiration of isolated skeletal muscle mitochondria after 1 h of moderate cycling in sedentary adults. https://doi.org/10.1249/mss.0000000000002615 | Human | Skeletal muscle | ||
| Theall 2021 Physiol Rep | 2021 | Theall B, Stampley J, Cho E, Granger J, Johannsen NM, Irving BA, Spielmann G (2021) Impact of acute exercise on peripheral blood mononuclear cells nutrient sensing and mitochondrial oxidative capacity in healthy young adults. Physiol Rep 9:e15147. | Human | Blood cells | ||
| Apostolopoulou 2021 Sci Adv | 2021 | Apostolopoulou M, Mastrototaro L, Hartwig S, Pesta D, Straßburger K, de Filippo E, Jelenik T, Karusheva Y, Gancheva S, Markgraf D, Herder C, Nair KS, Reichert AS, Lehr S, Müssig K, Al-Hasani H, Szendroedi J, Roden M (2021) Metabolic responsiveness to training depends on insulin sensitivity and protein content of exosomes in insulin-resistant males. https://doi.org/10.1126/sciadv.abi9551 | Human | Skeletal muscle | Diabetes | |
| Pharaoh 2021 JCSM Rapid Commun | 2021 | Pharaoh G, Brown J, Ranjit R, Ungvari Z, Van Remmen H (2021) Reduced adenosine diphosphate sensitivity in skeletal muscle mitochondria increases reactive oxygen species production in mouse models of aging and oxidative stress but not denervation. https://doi.org/10.1002/rco2.29 | Mouse | Skeletal muscle | ||
| Cheng 2021 Comp Biochem Physiol B Biochem Mol Biol | 2021 | Cheng H, Munro D, Huynh K, Pamenter ME (2021) Naked mole-rat skeletal muscle mitochondria exhibit minimal functional plasticity in acute or chronic hypoxia. Comp Biochem Physiol B Biochem Mol Biol 255:110596. | Other mammals | Skeletal muscle | Hypoxia Oxidative stress;RONS | |
| Christiansen 2021 Sci Rep | 2021 | Christiansen LB, Dohlmann TL, Ludvigsen TP, Parfieniuk E, Ciborowski M, Szczerbinski L, Kretowski A, Desler C, Tiano L, Orlando P, Martinussen T, Olsen LH, Larsen S (2021) Atorvastatin impairs liver mitochondrial function in obese Göttingen Minipigs but heart and skeletal muscle are not affected. Sci Rep 11:2167. | Pig | Heart Skeletal muscle Liver | ||
| Komlodi 2021 BEC AmR-O2 | 2021 | Komlódi T, Sobotka O, Gnaiger E (2021) Facts and artefacts on the oxygen dependence of hydrogen peroxide production using Amplex UltraRed. Bioenerg Commun 2021.4. https://doi.org/10.26124/bec:2021-0004 | Saccharomyces cerevisiae | Other cell lines | Oxidative stress;RONS Hypoxia | |
| Dall 2021 J Biol Chem | 2021 | Dall M, Hassing AS, Niu L, Nielsen TS, Ingerslev LR, Sulek K, Trammell SAJ, Gillum MP, Barrès R, Larsen S, Poulsen SS, Mann M, Ørskov C, Treebak JT (2021) Hepatocyte-specific perturbation of NAD+ biosynthetic pathways in mice induces reversible nonalcoholic steatohepatitis-like phenotypes. J Biol Chem 297:101388. | Mouse | Liver | Other | |
| Vandenberg 2021 Neurochem Int | 2021 | Vandenberg GG, Dawson NJ, Head A, Scott GR, Scott AL (2021) Astrocyte-mediated disruption of ROS homeostasis in Fragile X mouse model. Neurochem Int 146:105036. | Mouse | Nervous system | Autism | |
| Yardeni 2021 Proc Natl Acad Sci U S A | 2021 | Yardeni T, Cristancho AG, McCoy AJ, Schaefer PM, McManus MJ, Marsh ED, Wallace DC (2021) An mtDNA mutant mouse demonstrates that mitochondrial deficiency can result in autism endophenotypes. Proc Natl Acad Sci U S A 118:e2021429118. | Mouse | Nervous system | Autism | |
| Sarabhai 2021 Diabetologia | 2021 | Sarabhai T, Koliaki C, Mastrototaro L, Kahl S, Pesta D, Apostolopoulou M, Wolkersdorfer M, Bönner AC, Bobrov P, Markgraf DF, Herder C, Roden M (2021) Dietary palmitate and oleate differently modulate insulin sensitivity in human skeletal muscle. Diabetologia 65:301-14. | Human | Skeletal muscle | Diabetes | |
| Hansen 2021 Physiol Rep | 2021 | Hansen C, Olsen K, Pilegaard H, Bangsbo J, Gliemann L, Hellsten Y (2021) High metabolic substrate load induces mitochondrial dysfunction in rat skeletal muscle microvascular endothelial cells. Physiol Rep 9:e14855. | Rat | Skeletal muscle Endothelial;epithelial;mesothelial cell | ||
| Furihata 2021 Commun Biol | 2021 | Furihata T, Takada S, Kakutani N, Maekawa S, Tsuda M, Matsumoto J, Mizushima W, Fukushima A, Yokota T, Enzan N, Matsushima S, Handa H, Fumoto Y, Nio-Kobayashi J, Iwanaga T, Tanaka S, Tsutsui H, Sabe H, Kinugawa S (2021) Cardiac-specific loss of mitoNEET expression is linked with age-related heart failure. Commun Biol 4:138. | Mouse | Heart | Aging;senescence Cardiovascular | |
| Riguet 2021 Nat Commun | 2021 | Riguet N, Mahul-Mellier AL, Maharjan N, Burtscher J, Croisier M, Knott G, Hastings J, Patin A, Reiterer V, Farhan H, Nasarov S, Lashuel HA (2021) Nuclear and cytoplasmic huntingtin inclusions exhibit distinct biochemical composition, interactome and ultrastructural properties. Nat Commun 12:6579. | Human | HEK | Neurodegenerative | |
| Flockhart 2021 Cell Metab | 2021 | Flockhart M, Nilsson LC, Tais S, Ekblom B, Apro W, Larsen FJ (2021) Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab 33:957-70. | Human | Skeletal muscle | ||
| Marrocco 2020 Thesis | 2020 | Marrocco A (2020) Alterations of CCSP expression and macrophages metabolism in the development of silica-induced pulmonary inflammation and fibrosis. PhD Thesis 108. | Mouse | Macrophage-derived | Other | |
| Duong 2020 Mitochondrion | 2020 | Duong QV, Hoffman A, Zhong K, Dessinger MJ, Zhang Y, Bazil JN (2020) Calcium overload decreases net free radical emission in cardiac mitochondria. Mitochondrion 51:126-39. | Guinea pig | Heart | Ischemia-reperfusion Oxidative stress;RONS | |
| Falcao-Tebas 2020 J Physiol | 2020 | Falcão-Tebas F, Marin EC, Kuang J, Bishop DJ, McConell GK (2020) Maternal exercise attenuates the lower skeletal muscle glucose uptake and insulin secretion caused by paternal obesity in female adult rat offspring. J Physiol 598:4251-70. | Rat | Skeletal muscle | Obesity | |
| Charles 2020 Nanomedicine (Lond) | 2020 | Charles C, Cohen-Erez I, Kazaoka B, Melnikov O, Stein DE, Sensenig R, Rapaport H, Orynbayeva Z (2020) Mitochondrial responses to organelle-specific drug delivering nanoparticles composed of polypeptide and peptide complexes. Nanomedicine (Lond) 15:2917-32. | Human | Endothelial;epithelial;mesothelial cell | ||
| Burtscher 2020 eNeuro | 2020 | Burtscher J, Copin JC, Sandi C, Lashuel HA (2020) Pronounced α-synuclein pathology in a seeding-based mouse model is not sufficient to induce mitochondrial respiration deficits in the striatum and amygdala. eNeuro 7:ENEURO.0110-20.2020. | Mouse | Nervous system | Parkinson's | |
| Ehrlicher 2020 FASEB J | 2020 | Ehrlicher SE, Stierwalt HD, Miller BF, Newsom SA, Robinson MM (2020) Mitochondrial adaptations to exercise do not require Bcl2-mediated autophagy but occur with BNIP3/Parkin activation. FASEB J 34:4602-18. | Mouse | Skeletal muscle | ||
| Buch 2020 FASEB J | 2020 | Buch BT, Halling JF, Ringholm S, Gudiksen A, Kjøbsted R, Olsen MA, Wojtaszewski JFP, Pilegaard H (2020) Colchicine treatment impairs skeletal muscle mitochondrial function and insulin sensitivity in an age-specific manner. FASEB J 34:8653-70. | Mouse | Skeletal muscle | Aging;senescence | |
| Silva-Rodrigues 2020 Free Radic Biol Med | 2020 | Silva-Rodrigues T, De-Souza-Ferreira E, Machado CM, Cabral-Braga B, Rodrigues-Ferreira C, Galina A (2020) Hyperglycemia in a Type 1 Diabetes Mellitus model causes a shift in mitochondria coupled-glucose phosphorylation and redox metabolism in rat brain. Free Radic Biol Med 160:796-806. | Rat | Nervous system | Oxidative stress;RONS | Diabetes |
| Smith 2020 J Biol Chem | 2020 | Smith CD, Schmidt CA, Lin CT, Fisher-Wellman KH, Neufer PD (2020) Flux through mitochondrial redox circuits linked to nicotinamide nucleotide transhydrogenase generates counterbalance changes in energy expenditure. J Biol Chem 295:16207-16. | Mouse | Skeletal muscle | Oxidative stress;RONS | |
| Marquez 2020 J Am Heart Assoc | 2020 | Marquez AM, Morgan RW, Ko T, Landis WP, Hefti MM, Mavroudis CD, McManus MJ, Karlsson M, Starr J, Roberts AL, Lin Y, Nadkarni V, Licht DJ, Berg RA, Sutton RM, Kilbaugh TJ (2020) Oxygen exposure during cardiopulmonary resuscitation is associated with cerebral oxidative injury in a randomized, blinded, controlled, preclinical trial. J Am Heart Assoc 9:015032. | Pig | Nervous system | Cardiovascular | |
| Dulac 2020 J Physiol | 2020 | Dulac M, Leduc-Gaudet JP, Reynaud O, Ayoub MB, Guérin A, Finkelchtein M, Hussain SN, Gouspillou G (2020) Drp1 knockdown induces severe muscle atrophy and remodelling, mitochondrial dysfunction, autophagy impairment and denervation . J Physiol 598:3691-710. | Mouse | Skeletal muscle | ||
| Makrecka-Kuka 2020 Sci Rep | 2020 | Makrecka-Kuka M, Dimitrijevs P, Domracheva I, Jaudzems K, Dambrova M, Arsenyan P (2020) Fused isoselenazolium salts suppress breast cancer cell growth by dramatic increase in pyruvate-dependent mitochondrial ROS production. https://doi.org/10.1038/s41598-020-78620-8. | Mouse | Endothelial;epithelial;mesothelial cell | Cancer | |
| Baxter 2020 Elife | 2020 | Baxter M, Voronkov M, Poolman T, Galli G, Pinali C, Goosey L, Knight A, Krakowiak K, Maidstone R, Iqbal M, Zi M, Prehar S, Cartwright EJ, Gibbs J, Matthews LC, Adamson AD, Humphreys NE, Rebelo-Guiomar P, Minczuk M, Bechtold DA, Loudon A, Ray D (2020) Cardiac mitochondrial function depends on BUD23 mediated ribosome programming. Elife 9:e50705. | Mouse | Heart | ||
| Olsen 2020 J Physiol | 2020 | Olsen LN, Hoier B, Hansen CV, Leinum M, Carter HH, Jorgensen TS, Bangsbo J, Hellsten Y (2020) Angiogenic potential is reduced in skeletal muscle of aged women. J Physiol 598:5149-64. | Human | Endothelial;epithelial;mesothelial cell | Aging;senescence | |
| Li Puma 2020 Am J Physiol Regul Integr Comp Physiol | 2020 | Li Puma LC, Hedges M, Heckman JM, Mathias AB, Engstrom MR, Brown AB, Chicco AJ (2020) Experimental oxygen concentration influences rates of mitochondrial hydrogen peroxide release from cardiac and skeletal muscle preparations. Am J Physiol Regul Integr Comp Physiol 318:972-80. | Mouse | Heart Skeletal muscle | Oxidative stress;RONS | |
| Souza da Silva 2020 Mol Neurobiol | 2020 | Souza da Silva J, Nonose Y, Rohden F, Lukasewicz Ferreira PC, Fontella FU, Rocha A, Wigner Brochier A, Vieira Apel R, de Lima TM, Seminotti B, Amaral AU, Galina A, Souza DO (2020) Guanosine neuroprotection of presynaptic mitochondrial calcium homeostasis in a mouse study with amyloid-β oligomers. Mol Neurobiol 57:4790-809. | Mouse | Nervous system | Alzheimer's | |
| Le 2020 J Biol Chem | 2020 | Le CH, Benage LG, Specht KS, Li Puma LC, Mulligan CM, Heuberger AL, Prenni JE, Claypool SM, Chatfield KC, Sparagna GC, Chicco AJ (2020) Tafazzin deficiency impairs CoA-dependent oxidative metabolism in cardiac mitochondria. J Biol Chem 295:12485-97. | Human | Heart | Aging;senescence | |
| Hafstad 2020 Antioxidants (Basel) | 2020 | Hafstad AD, Hansen SS, Lund J, Santos CXC, Boardman NT, Shah AM, Aasum E (2020) NADPH oxidase 2 mediates myocardial oxygen wasting in obesity. Antioxidants (Basel) 9:E171. | Mouse | Heart | Oxidative stress;RONS | Obesity |
| Chausse 2020 Brain Behav Immun | 2020 | Chausse B, Lewen A, Poschet G, Kann O (2020) Selective inhibition of mitochondrial respiratory complexes controls the transition of microglia into a neurotoxic phenotype in situ. Brain Behav Immun 88:802-14. | Mouse | Nervous system | ||
| Avila-Rojas 2020 Food Chem Toxicol | 2020 | Avila-Rojas SH, Aparicio-Trejo OE, Briones-Herrera A, Medina-Campos ON, Reyes-Fermín LM, Martínez-Klimova E, León-Contreras JC, Hernández-Pando R, Tapia E, Pedraza-Chaverri J (2020) Alterations in mitochondrial homeostasis in a potassium dichromate model of acute kidney injury and their mitigation by curcumin. Food Chem Toxicol 145:111774. | Rat | Kidney | ||
| Hartmann 2020 Free Radic Res | 2020 | Hartmann DD, Gonçalves DF, Da Rosa PC, Martins RP, Courtes AA, Franco JL, A Soares FA, Puntel GO (2020) A single muscle contusion promotes an immediate alteration in mitochondrial bioenergetics response in skeletal muscle fibres with different metabolism. Free Radic Res 54:137-49. | Rat | Skeletal muscle | Other | |
| Fuller 2020 J Appl Physiol (1985) | 2020 | Fuller KNZ, McCoin CS, Allen J, Bell-Glenn S, Koestler DC, Dorn Ii GW, Thyfault JP (2020) Sex and BNIP3 genotype, rather than acute lipid injection, modulate hepatic mitochondrial function and steatosis risk in mice. J Appl Physiol (1985) 128:1251-61. | Mouse | Liver | Oxidative stress;RONS | |
| Hedges 2020 Biosci Rep | 2020 | Hedges CP, Pham T, Shetty B, Masson SWC, Hickey AJR, Shepherd PR, Merry TL (2020) Prolonged treatment with a PI3K p110α inhibitor causes sex- and tissue-dependent changes in antioxidant content, but does not affect mitochondrial function. https://doi.org/10.1042/bsr20201128 | Mouse | Skeletal muscle Liver | ||
| Pharaoh 2020 Sci Rep | 2020 | Pharaoh G, Brown JL, Sataranatarajan K, Kneis P, Bian J, Ranjit R, Hadad N, Georgescu C, Rabinovitch P, Ran Q, Wren JD, Freeman W, Kinter M, Richardson A, Van Remmen H (2020) Targeting cPLA2 derived lipid hydroperoxides as a potential intervention for sarcopenia. Sci Rep 10:13968. | Mouse | Skeletal muscle | Oxidative stress;RONS | Aging;senescence |
| Davidson 2020 Circ Res | 2020 | Davidson MT, Grimsrud P, Lai L, Draper J, Fisher-Wellman KH, Narowski TM, Koves TR, Kelly DP, Muoio DM (2020) Extreme acetylation of the cardiac mitochondrial proteome does not promote heart failure. Circ Res 127:1094-108. | Mouse | Heart | ||
| Purhonen 2020 Nat Commun | 2020 | Purhonen J, Grigorjev V, Ekiert R, Aho N, Rajendran J, Pietras R, Truvé K, Wikström M, Sharma V, Osyczka A, Fellman V, Kallijärvi J (2020) A spontaneous mitonuclear epistasis converging on Rieske Fe-S protein exacerbates complex III deficiency in mice. Nat Commun 11:322. | Mouse | Liver Kidney | Mitochondrial disease | |
| Brunetta 2020 J Physiol | 2020 | Brunetta HS, Politis-Barber V, Petrick HL, Dennis KMJH, Kirsh AJ, Barbeau PA, Nunes EA, Holloway GP (2020) Nitrate attenuates HFD-induced glucose intolerance in association with reduced epididymal adipose tissue inflammation and mitochondrial ROS emission. J Physiol 598:3357-71. | Mouse | Fat | Obesity | |
| Kunz 2020 J Biol Chem | 2020 | Kunz HE, Dorschner JM, Berent TE, Meyer T, Wang X, Jatoi A, Kumar R, Lanza IR (2020) Methylarginine metabolites are associated with attenuated muscle protein synthesis in cancer-associated muscle wasting. J Biol Chem 295:17441-59. | Mouse | Skeletal muscle | Cancer | |
| Mahul-Mellier 2020 Proc Natl Acad Sci U S A | 2020 | Mahul-Mellier AL, Burtscher J, Maharjan N, Weerens L, Croisier M, Kuttler F, Leleu M, Knott GW, Lashuel HA (2020) The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci U S A 117:4971-82. | Mouse | Nervous system | Parkinson's | |
| Szibor 2020 J Cell Mol Med | 2020 | Szibor M, Schreckenberg R, Gizatullina Z, Dufour E, Wiesnet M, Dhandapani PK, Debska-Vielhaber G, Heidler J, Wittig I, Nyman TA, Gaertner U, Hall AR, Pell V, Viscomi C, Krieg T, Murphy MP, Braun T, Gellerich FN, Schlueter KD, Jacobs HT(2020) Respiratory chain signalling is essential for adaptive remodelling following cardiac ischaemia. J Cell Mol Med 24:3534-48. | Mouse | Heart | Ischemia-reperfusion | |
| Zussman 2020 J Autoimmun | 2020 | Zussman R, Xu LY, Damani T, Groom KM, Chen Q, Seers B, Viall CA, Chamley LW, Hickey A (2020) Antiphospholipid antibodies can specifically target placental mitochondria and induce ROS production. J Autoimmun 111:102437. | Human | Genital | Oxidative stress;RONS | |
| Abid 2020 FASEB J | 2020 | Abid H, Ryan ZC, Delmotte P, Sieck GC, Lanza IR (2020) Extramyocellular interleukin-6 influences skeletal muscle mitochondrial physiology through canonical JAK/STAT signaling pathways. FASEB J 34:14458-72. | Mouse | Skeletal muscle | Oxidative stress;RONS | |
| Hernansanz-Agustin 2020 Nature | 2020 | Hernansanz-Agustín P, Choya-Foces C, Carregal-Romero S, Ramos E, Oliva T, Villa-Piña T, Moreno L, Izquierdo-Álvarez A, Cabrera-García JD, Cortés A, Lechuga-Vieco AV, Jadiya P, Navarro E, Parada E, Palomino-Antolín A, Tello D, Acín-Pérez R, Rodríguez-Aguilera JC, Navas P, Cogolludo Á, López-Montero I, Martínez-Del-Pozo Á, Egea J, López MG, Elrod JW, Ruíz-Cabello J, Bogdanova A, Enríquez JA, Martínez-Ruiz A (2020) Na+ controls hypoxic signalling by the mitochondrial respiratory chain. Nature 586:287-91. | Rat | Heart | Oxidative stress;RONS Hypoxia | |
| Mendham 2020 Sci Rep | 2020 | Mendham Amy E, Larsen Steen, George Cindy, Adams Kevin, Hauksson Jon, Olsson Tommy, Fortuin-de Smidt Melony C, Nono Nankam Pamela A, Hakim Olah, Goff Louise M, Pheiffer Carmen, Goedecke Julia H (2020) Exercise training results in depot-specific adaptations to adipose tissue mitochondrial function. Sci Rep 10:3785. | Fat | Diabetes Obesity | ||
| Pham 2020 Eur J Appl Physiol | 2020 | Pham T, MacRae CL, Broome SC, D'souza RF, Narang R, Wang HW, Mori TA, Hickey AJR, Mitchell CJ, Merry TL (2020) MitoQ and CoQ10 supplementation mildly suppresses skeletal muscle mitochondrial hydrogen peroxide levels without impacting mitochondrial function in middle-aged men. Eur J Appl Physiol 120:1657-69. | Human | Skeletal muscle | Oxidative stress;RONS | |
| Kupats 2020 Oxid Med Cell Longev | 2020 | Kupats E, Stelfa G, Zvejniece B, Grinberga S, Vavers E, Makrecka-Kuka M, Svalbe B, Zvejniece L, Dambrova M (2020) Mitochondrial-protective effects of R-phenibut after experimental traumatic brain injury. Oxid Med Cell Longev 2020:9364598. | Mouse | Nervous system | Other | |
| Dirks 2020 J Physiol | 2020 | Dirks ML, Miotto PM, Goossens GH, Senden JM, Petrick HL, van Kranenburg J, van Loon LJC, Holloway GP (2020) Short-term bed rest-induced insulin resistance cannot be explained by increased mitochondrial H2O2 emission. J Physiol 598:123-37. | Human | Skeletal muscle | Oxidative stress;RONS | |
| Aparicio-Trejo 2020 Free Radic Biol Med | 2020 | Aparicio-Trejo OE, Avila-Rojas SH, Tapia E, Rojas-Morales P, León-Contreras JC, Martínez-Klimova E, Hernández-Pando R, Sánchez-Lozada LG, Pedraza-Chaverri J (2020) Chronic impairment of mitochondrial bioenergetics and β-oxidation promotes experimental AKI-to-CKD transition induced by folic acid. Free Radic Biol Med 154:18-32. | Rat | Kidney | Other | |
| Bundgaard 2020 J Exp Biol | 2020 | Bundgaard A, Qvortrup K, Rasmussen LJ, Fago A (2020) Turtles maintain mitochondrial integrity but reduce mitochondrial respiratory capacity in the heart after cold acclimation and anoxia. J Exp Biol 222:jeb200410. | Amphibians | Heart | ||
| Hernansanz-Agustin 2019 bioRxiv | 2019 | Hernansanz-Agustín P, Choya-Foces C, Carregal-Romero S, Ramos E, Oliva T, Villa-Piña T, Moreno L, Izquierdo-Álvarez A, JCabrera-García JD, Cortés A, Lechuga-Vieco AV, Jadiya P, Navarro E, Parada E, Palomino-Antolín A, Tello D, Acín-Pérez R, Rodríguez-Aguilera JC, Navas P, Cogolludo A, López-Montero I, Martínez-del-Pozo A, Egea J, López MG, Elrod JW, Ruiz-Cabello J, Bogdanova A, Enríquez JA, Martínez-Ruiz A (2019) Mitochondrial Na+ import controls oxidative phosphorylation and hypoxic redox signalling. bioRxiv doi: https://doi.org/10.1101/385690. | Rat Bovines | Heart | Hypoxia | |
| Lefranc 2019 Hypertension | 2019 | Lefranc C, Friederich-Persson M, Braud L, Palacios-Ramirez R, Karlsson S, Boujardine N, Motterlini R, Jaisser F, Nguyen Dinh Cat A (2019) MR (mineralocorticoid receptor) induces adipose tissue senescence and mitochondrial dysfunction leading to vascular dysfunction in obesity. Hypertension 73:458-68. | Mouse | Fat | Aging;senescence Obesity | |
| Power 2019 PLoS One | 2019 | Power AS, Norman R, Jones TLM, Hickey AJ, Ward ML (2019) Mitochondrial function remains impaired in the hypertrophied right ventricle of pulmonary hypertensive rats following short duration metoprolol treatment. PLoS One 14:e0214740. | Rat | Heart | Cardiovascular | |
| Desquiret-Dumas 2019 Biochim Biophys Acta Mol Basis Dis | 2019 | Desquiret-Dumas V, Leman G, Wetterwald C, Chupin S, Lebert A, Khiati S, Le Mao M, Geffroy G, Kane MS, Chevrollier A, Goudenege D, Gadras C, Tessier L, Barth M, Leruez S, Amati-Bonneau P, Henrion D, Bonneau D, Procaccio V, Reynier P, Lenaers G, Gueguen N (2019) Warburg-like effect is a hallmark of complex I assembly defects. Biochim Biophys Acta Mol Basis Dis 1865:2475-89. | Human | Fibroblast | Other | |
| McBride 2019 Arch Biochem Biophys | 2019 | McBride S, Wei-LaPierre L, McMurray F, MacFarlane M, Qiu X, Patten DA, Dirksen RT, Harper ME (2019) Skeletal muscle mitoflashes, pH, and the role of uncoupling protein-3. Arch Biochem Biophys 663:239-48. | Mouse | Skeletal muscle | Oxidative stress;RONS | |
| Seo 2019 Exp Neurol | 2019 | Seo JH, Park HS, Park SS, Kim CJ, Kim DH, Kim TW (2019) Physical exercise ameliorates psychiatric disorders and cognitive dysfunctions by hippocampal mitochondrial function and neuroplasticity in post-traumatic stress disorder. Exp Neurol 322:113043. | Rat | Nervous system | Other | |
| Laouafa 2019 Acta Physiol (Oxf) | 2019 | Laouafa S, Roussel D, Marcouiller F, Soliz J, Gozal D, Bairam A, Joseph V (2019) Roles of oestradiol receptor alpha and beta against hypertension and brain mitochondrial dysfunction under intermittent hypoxia in female rats. Acta Physiol (Oxf) 226:e13255. | Rat | Nervous system | Hypoxia | |
| Pharaoh 2019 Mol Neurobiol | 2019 | Pharaoh G, Owen D, Yeganeh A, Premkumar P, Farley J, Bhaskaran S, Ashpole N, Kinter M, Van Remmen H, Logan S (2019) Disparate central and peripheral effects of circulating IGF-1 deficiency on tissue mitochondrial function. Mol Neurobiol 57:1317-31. | Mouse | Skeletal muscle Nervous system Fat | Aging;senescence | |
| Miotto 2019 FASEB J | 2019 | Miotto PM, McGlory C, Bahniwal R, Kamal M, Phillips SM, Holloway GP (2019) Supplementation with dietary ω-3 mitigates immobilization-induced reductions in skeletal muscle mitochondrial respiration in young women. FASEB J 33:8232-40. | Human | Skeletal muscle | ||
| Bundgaard 2019 Sci Rep | 2019 | Bundgaard A, James AM, Gruszczyk AV, Martin J, Murphy MP, Fago A (2019) Metabolic adaptations during extreme anoxia in the turtle heart and their implications for ischemia-reperfusion injury. Sci Rep 9:2850. | Mouse Reptiles | Heart | Ischemia-reperfusion Oxidative stress;RONS | |
| Holsgrove 2019 Thesis | 2019 | Holsgrove A (2019) The effect of temperature on cardiac energetics in the rainbow trout, Onchorhynchus mykiss. Phd Thesis 166. | Fishes | Heart | ||
| Robinson 2019 Am J Physiol Cell Physiol | 2019 | Robinson MM, Sather BK, Burney ER, Ehrlicher SE, Stierwalt HD, Franco MC, Newsom SA (2019) Robust intrinsic differences in mitochondrial respiration and H2O2 emission between L6 and C2C12 cells. Am J Physiol Cell Physiol 317:C339-C347. | Mouse Rat | Skeletal muscle | ||
| Stepanova 2019 Antioxid Redox Signal | 2019 | Stepanova Anna, Sosunov S, Niatsetskaya Z, Konrad Csaba, Starkov AA, Manfredi G, Wittig I, Ten V, Galkin Alexander (2019) Redox-dependent loss of flavin by mitochondrial complex I in brain ischemia/reperfusion injury. Antioxid Redox Signal 31:608-22. | Mouse | Nervous system | Ischemia-reperfusion | |
| Ahn 2019 J Cachexia Sarcopenia Muscle | 2019 | Ahn B, Ranjit R, Premkumar P, Pharaoh G, Piekarz KM, Matsuzaki S, Claflin DR, Riddle K, Judge J, Bhaskaran S, Satara Natarajan K, Barboza E, Wronowski B, Kinter M, Humphries KM, Griffin TM, Freeman WM, Richardson A, Brooks SV, Van Remmen H (2019) Mitochondrial oxidative stress impairs contractile function but paradoxically increases muscle mass via fiber branching. J Cachexia Sarcopenia Muscle 10:411-28. | Mouse | Skeletal muscle | Oxidative stress;RONS | |
| Hedges 2019 Comp Biochem Physiol A Mol Integr Physiol | 2019 | Hedges CP, Wilkinson RT, Devaux JBL, Hickey AJR (2019) Hymenoptera flight muscle mitochondrial function: Increasing metabolic power increases oxidative stress. Comp Biochem Physiol A Mol Integr Physiol 230:115-21. | Other invertebrates | Skeletal muscle | Oxidative stress;RONS | |
| Monteiro 2019 J Bioenerg Biomembr | 2019 | Monteiro J, Assis-de-Lemos G, de-Souza-Ferreira E, Marques AM, Neves GA, Silveira MS, Galina A (2019) Energization by multiple substrates and calcium challenge reveal dysfunctions in brain mitochondria in a model related to acute psychosis. J Bioenerg Biomembr 52:1-15. | Mouse | Nervous system | Oxidative stress;RONS | Other |
| Cannon 2019 J Appl Physiol (1985) | 2019 | Cannon DT, Rodewohl L, Adams V, Breen EC, Bowen TS (2019) Skeletal myofiber VEGF deficiency leads to mitochondrial, structural and contractile alterations in mouse diaphragm. J Appl Physiol (1985) 127:1360-69. | Mouse | Skeletal muscle | ||
| Munro 2019 Aging Cell | 2019 | Munro D, Baldy C, Pamenter ME, Treberg JR (2019) The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses. Aging Cell 18:e12916. | Mouse Other mammals | Heart Skeletal muscle | Oxidative stress;RONS | Aging;senescence |
| Isei 2019 Free Radic Biol Med | 2019 | Isei MO, Kamunde C (2019) Effects of copper and temperature on heart mitochondrial hydrogen peroxide production. Free Radic Biol Med 147:114-28. | Fishes | Heart | Oxidative stress;RONS | |
| Shirakawa 2019 Sci Rep | 2019 | Shirakawa R, Yokota T, Nakajima T, Takada S, Yamane M, Furihata T, Maekawa S, Nambu H, Katayama T, Fukushima A, Saito A, Ishimori N, Dela F, Kinugawa S, Anzai T (2019) Mitochondrial reactive oxygen species generation in blood cells is associated with disease severity and exercise intolerance in heart failure patients. Sci Rep 9:14709. | Human | Blood cells | Oxidative stress;RONS | Cardiovascular |
| Szibor 2019 Biochim Biophys Acta Bioenerg | 2019 | Szibor Marten, Gainutdinov Timur, Fernandez-Vizarra Erika, Dufour Eric, Gizatullina Zemfira, Debska-Vielhaber Grazyna, Heidler Juliana, Wittig Ilka, Viscomi Carlo, Gellerich Frank Norbert, Moore Anthony L (2019) Bioenergetic consequences from xenotopic expression of a tunicate AOX in mouse mitochondria: switch from RET and ROS to FET. Biochim Biophys Acta Bioenerg 1861:148137. | Mouse | Heart | ||
| Scheiber 2019 Exp Mol Med | 2019 | Scheiber D, Jelenik T, Zweck E, Horn P, Schultheiss HP, Lassner D, Boeken U, Saeed D, Kelm M, Roden M, Westenfeld R, Szendroedi J (2019) High-resolution respirometry in human endomyocardial biopsies shows reduced ventricular oxidative capacity related to heart failure. Exp Mol Med 51:16. | Human | Heart | Cardiovascular | |
| Ruegsegger 2019 JCI Insight | 2019 | Ruegsegger GN, Vanderboom PM, Dasari S, Klaus KA, Kabiraj P, McCarthy CB, Lucchinetti CF, Nair KS (2019) Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight 4:130681. | Mouse | Nervous system | Diabetes | |
| McMurray 2019 FASEB J | 2019 | McMurray F, MacFarlane M, Kim K, Patten DA, Wei-LaPierre L, Fullerton MD, Harper ME (2019) Maternal diet-induced obesity alters muscle mitochondrial function in offspring without changing insulin sensitivity. FASEB J 33:13515-26. | Mouse | Skeletal muscle | Oxidative stress;RONS | Diabetes Obesity |
| Ederle 2019 J Clin Med | 2019 | Ederle C, Charles AL, Khayath N, Poirot A, Meyer A, Clere-Jehl R, Andres E, De Blay F, Geny B (2019) Mitochondrial function in peripheral blood mononuclear cells (PBMC) is enhanced, together with increased reactive oxygen species, in severe asthmatic patients in exacerbation. J Clin Med 8:E1613. | Human | Blood cells | Oxidative stress;RONS | Other |
| Spinazzi 2019 Proc Natl Acad Sci U S A | 2019 | Spinazzi M, Radaelli E, Horré K, Arranz AM, Gounko NV, Agostinis P, Maia TM, Impens F, Morais VA, Lopez-Lluch G, Serneels L, Navas P, De Strooper B (2019) PARL deficiency in mouse causes Complex III defects, coenzyme Q depletion, and Leigh-like syndrome. Proc Natl Acad Sci U S A 116:277-86. 10.1073/pnas.1811938116 | Mouse | Nervous system | Neurodegenerative | |
| Halling 2019 Am J Physiol Endocrinol Metab | 2019 | Halling JF, Jessen H, Nøhr-Meldgaard J, Thiellesen Buch B, Masselkhi Christensen N, Gudiksen A, Ringholm S, Neufer PD, Prats C, Pilegaard H (2019) PGC-1α regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am J Physiol Endocrinol Metab 317:E513-E525. | Mouse | Skeletal muscle | Aging;senescence | |
| Perry 2019 J Mol Cell Cardiol | 2019 | Perry JB, Davis GN, Allen ME, Makrecka-Kuka M, Dambrova M, Grange RW, Shaikh SR, Brown DA (2019) Cardioprotective effects of idebenone do not involve ROS scavenging: Evidence for mitochondrial complex I bypass in ischemia/reperfusion injury. J Mol Cell Cardiol 135:160-171. | Rat | Heart | Ischemia-reperfusion | |
| Courtes 2019 Biomed Pharmacother | 2019 | Courtes AA, de Carvalho NR, Gonçalves DF, Hartmann DD, da Rosa PC, Dobrachinski F, Franco JL, de Souza DOG, Soares FAA (2019) Guanosine protects against Ca2+-induced mitochondrial dysfunction in rats. Biomed Pharmacother 111:1438-46. | Rat | Liver | ||
| Cooper 2019 Exp Physiol | 2019 | Cooper MA, McCoin C, Pei D, Thyfault JP, Koestler D, Wright DE (2019) Reduced mitochondrial reactive oxygen species production in peripheral nerves of mice fed a ketogenic diet. Exp Physiol 103:1206-12. | Mouse | Nervous system | ||
| Axton 2019 J Nutr | 2019 | Axton ER, Beaver LM, St Mary L, Truong L, Logan CR, Spagnoli S, Prater MC, Keller RM, Garcia-Jaramillo M, Ehrlicher SE, Stierwalt HD, Newsom SA, Robinson MM, Tanguay RL, Stevens JF, Hord NG (2019) Treatment with nitrate, but not nitrite, lowers the oxygen cost of exercise and decreases glycolytic intermediates while increasing fatty acid metabolites in exercised zebrafish. J Nutr 00:1–13. | Zebrafish | Skeletal muscle | ||
| Rajendran 2019 EMBO Mol Med | 2019 | Rajendran J, Purhonen J, Tegelberg S, Smolander OP, Mörgelin M, Rozman J, Gailus-Durner V, Fuchs H, Hrabe de Angelis M, Auvinen P, Mervaala E, Jacobs HT, Szibor M, Fellman V, Kallijärvi J (2019) Alternative oxidase-mediated respiration prevents lethal mitochondrial cardiomyopathy. EMBO Mol Med 11:e9456. | Mouse | Liver Kidney Heart | Mitochondrial disease | |
| Ruegsegger 2019 FASEB J | 2019 | Ruegsegger GN, Manjunatha S, Summer P, Gopala S, Zabeilski P, Dasari S, Vanderboom PM, Lanza IR, Klaus KA, Nair KS (2019) Insulin deficiency and intranasal insulin alter brain mitochondrial function: a potential factor for dementia in diabetes. FASEB J 33:4458-72. | Mouse | Nervous system | Oxidative stress;RONS | Diabetes |
| Jang 2018 J Med Toxicol | 2018 | Jang DH, Khatri UG, Mudan A, Love JS, Owiredu S, Eckmann DM (2018) Translational application of measuring mitochondrial functions in blood cells obtained from patients with acute poisoning. J Med Toxicol 14:144-51. | Human | Blood cells | Cardiovascular | |
| Aparicio-Trejo 2018 Free Radic Biol Med | 2018 | Aparicio-Trejo OE, Reyes-Fermín LM, Briones-Herrera A, Tapia E, León-Contreras JC, Hernández-Pando R, Sánchez-Lozada LG, Pedraza-Chaverri J (2018) Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic Biol Med 130:379-96. | Rat | Kidney | Oxidative stress;RONS | Other |
| Briones-Herrera 2018 Food Chem Toxicol | 2018 | Briones-Herrera A, Avila-Rojas SH, Aparicio-Trejo OE, Cristóbal M, León-Contreras JC, Hernández-Pando R, Pinzón E, Pedraza-Chaverri J, Sánchez-Lozada LG, Tapia E (2018) Sulforaphane prevents maleic acid-induced nephropathy by modulating renal hemodynamics, mitochondrial bioenergetics and oxidative stress. Food Chem Toxicol 115:185-97. | Rat | Kidney | Other | |
| Logan 2018 Thesis | 2018 | Logan C (2018) Nitrate and nitrite differentially affect respiration in zebrafish during exercise. Honors Baccalaureate of Science in Nutrition p44. | Zebrafish | Skeletal muscle | ||
| Trewin 2018 Am J Physiol Regul Integr Comp Physiol | 2018 | Trewin AJ, Parker L, Shaw CS, Hiam D, Garnham AP, Levinger I, McConell GK, Stepto NK (2018) Acute HIIE elicits similar changes in human skeletal muscle mitochondrial H2O2 release, respiration and cell signaling as endurance exercise even with less work. Am J Physiol Regul Integr Comp Physiol 315:R1003-R1016. | Human | Skeletal muscle | ||
| Jelenik 2018 Mol Metab | 2018 | Jelenik T, Dille M, Müller-Lühlhoff S, Kabra DG, Zhou Z, Binsch C, Hartwig S, Lehr S, Chadt A, Peters EMJ, Kruse J, Roden M, Al-Hasani H, Castañeda TR (2018) FGF21 regulates insulin sensitivity following long-term chronic stress. Mol Metab 16:126-38. | Mouse | Skeletal muscle | Other Diabetes | |
| Nielsen 2018 Neurosci Lett | 2018 | Nielsen B, Cejvanovic V, Wörtwein G, Hansen AR, Marstal KK, Weimann A, Bjerring PN, Dela F, Poulsen HE, Jørgensen MB (2018) Increased oxidation of RNA despite reduced mitochondrial respiration after chronic electroconvulsive stimulation of rat brain tissue. Neurosci Lett 690:1-5. | Rat | Nervous system | Other | |
| Scheiber 2018 J Cardiovasc Transl Res | 2018 | Scheiber D, Zweck E, Jelenik T, Horn P, Albermann S, Masyuk M, Boeken U, Saeed D, Kelm M Roden M, Szendroedi J, Westenfeld R (2018) Reduced myocardial mitochondrial ROS production in mechanically unloaded hearts. J Cardiovasc Transl Res 12:107-15. | Human | Heart | Cardiovascular | |
| Kim 2018 Free Radic Biol Med | 2018 | Kim M, Stepanova A, Niatsetskaya Z, Sosunov S, Arndt S, Murphy MP, Galkin A, Ten VS (2018) Attenuation of oxidative damage by targeting mitochondrial complex I in neonatal hypoxic-ischemic brain injury. Free Radic Biol Med 124:517-24. | Mouse | Nervous system | Ischemia-reperfusion Hypoxia | |
| Holloway 2018 Cell Rep | 2018 | Holloway GP, Holwerda AM, Miotto PM, Dirks ML, Verdijk LB, van Loon LJC (2018) Age-associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle. Cell Rep 22:2837–48. | Human | Skeletal muscle | Oxidative stress;RONS | Aging;senescence |
| Karlsson 2018 J Neurotrauma | 2018 | Karlsson M, Pukenas B, Chawla S, Ehinger JK, Plyler R, Stolow M, Gabello M, Hugerth M, Elmér E, Hansson MJ, Margulies S, Kilbaugh T (2018) Neuroprotective effects of cyclosporine in a porcine pre-clinical trial of focal traumatic brain injury. J Neurotrauma 36:14-24. | Pig | Nervous system | Other | |
| Schiffer 2018 PLoS One | 2018 | Schiffer TA, Christensen M, Gustafsson H, Palm F (2018) The effect of inactin on kidney mitochondrial function and production of reactive oxygen species. PLoS One 13:e0207728. | Rat | Kidney | Oxidative stress;RONS | |
| Mavroudis 2018 Eur J Cardiothorac Surg | 2018 | Mavroudis CD, Karlsson M, Ko T, Hefti M, Gentile JI, Morgan RW, Plyler R, Mensah-Brown KG, Boorady TW, Melchior RW, Rosenthal TM, Shade BC, Schiavo KL, Nicolson SC, Spray TL, Sutton RM, Berg RA, Licht DJ, Gaynor JW, Kilbaugh TJ (2018) Cerebral mitochondrial dysfunction associated with deep hypothermic circulatory arrest in neonatal swine. Eur J Cardiothorac Surg 54:162-68. | Pig | Nervous system | Ischemia-reperfusion Temperature | |
| Valentine 2018 J Gerontol A Biol Sci Med Sci | 2018 | Valentine JM, Li ME, Shoelson SE, Zhang N, Reddick RL, Musi N (2018) NFκB regulates muscle development and mitochondrial function. J Gerontol A Biol Sci Med Sci 75:647-53. | Mouse | Skeletal muscle | ||
| Stepanova 2018 J Cereb Blood Flow Metab | 2018 | Stepanova A, Konrad C, Guerrero-Castillo S, Manfredi G, Vannucci S, Arnold S, Galkin A (2018) Deactivation of mitochondrial complex I after hypoxia-ischemia in the immature brain. J Cereb Blood Flow Metab 39:1790-802. | Rat | Nervous system | Hypoxia Ischemia-reperfusion | |
| Hedges 2018 FASEB J | 2018 | Hedges CP, Bishop DJ, Hickey AJR (2018) Voluntary wheel running prevents the acidosis-induced decrease in skeletal muscle mitochondrial reactive oxygen species emission. FASEB J 33:4996-5004. | Rat | Skeletal muscle | Oxidative stress;RONS | |
| Burney 2018 Thesis | 2018 | Burney E (2018) Characterization of mitochondrial metabolism in L6 rat myoblasts. Bachelor's Thesis p50. | Rat | Other cell lines | ||
| Souza 2018 Sci Rep | 2018 | Souza RWA, Alves CRR, Medeiros A, Rolim N, Silva GJJ, Moreira JBN, Alves MN, Wohlwend M, Gebriel M, Hagen L, Sharma A, Koch LG, Britton SL, Slupphaug G, Wisløff U, Brum PC (2018) Differential regulation of cysteine oxidative post-translational modifications in high and low aerobic capacity. Sci Rep 8:17772. | Rat | Heart Skeletal muscle | ||
| Ahn 2018 Redox Biol | 2018 | Ahn B, Pharaoh G, Premkumar P, Huseman K, Ranjit R, Kinter M, Szweda L, Kiss T, Fulop G, Tarantini S, Csiszar A, Ungvari Z, Van Remmen H (2018) Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol 17:47-58. | Mouse | Skeletal muscle | Aging;senescence | |
| Dogan 2018 Cell Metab | 2018 | Dogan SA, Cerutti R, Benincá C, Brea-Calvo G, Jacobs HT, Zeviani M, Szibor M, Viscomi C (2018) Perturbed redox signaling exacerbates a mitochondrial myopathy. Cell Metab 28:764-77. | Mouse | Skeletal muscle | Mitochondrial disease | Myopathy |
| Komlodi 2018 Methods Mol Biol | 2018 | Komlodi T, Sobotka O, Krumschnabel G, Bezuidenhout N, Hiller E, Doerrier C, Gnaiger E (2018) Comparison of mitochondrial incubation media for measurement of respiration and hydrogen peroxide production. Methods Mol Biol 1782:137-55. | Human Mouse | Skeletal muscle HEK | ||
| Treberg 2018 Comp Biochem Physiol B Biochem Mol Biol | 2018 | Treberg JR, Braun K, Zacharias P, Kroeker K (2018) Multidimensional mitochondrial energetics: Application to the study of electron leak and hydrogen peroxide metabolism. Comp Biochem Physiol B Biochem Mol Biol 224:121-28. | Mouse Rat | Skeletal muscle | ||
| Dawson 2018 Acta Physiol (Oxf) | 2018 | Dawson NJ, Lyons SA, Henry DA, Scott GR (2018) Effects of chronic hypoxia on diaphragm function in deer mice native to high altitude. Acta Physiol (Oxf) 223:e13030. | Other mammals | Skeletal muscle | Hypoxia | |
| Campbell 2018 Free Radic Biol Med | 2018 | Campbell MD, Duan J, Samuelson AT, Gaffrey MJ, Wang L, Bammler TK, Moore RJ, White CC, Kavanagh TJ, Voss JG, Szeto HH, Rabinovitch PS, Qian WJ, Marcinek DJ (2018) Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol Med 134:268-81. | Mouse | Skeletal muscle | Oxidative stress;RONS | Aging;senescence |
| Kjeld 2018 PLoS One | 2018 | Kjeld T, Stride N, Gudiksen A, Hansen EG, Arendrup HC, Horstmann PF, Zerahn B, Jensen LT, Nordsborg N, Bejder J, Halling JF (2018) Oxygen conserving mitochondrial adaptations in the skeletal muscles of breath hold divers. PLoS One 13:e0201401. | Human | Skeletal muscle | ||
| Larsen 2018 Physiol Rep | 2018 | Larsen S, Lundby AM, Dandanell S, Oberholzer L, Keiser S, Andersen AB, Haider T, Lundby C (2018) Four days of bed rest increases intrinsic mitochondrial respiratory capacity in young healthy males. Physiol Rep 6:e13793. | Human | Skeletal muscle | ||
| Stepanova 2018 J Neurochem | 2018 | Stepanova A, Konrad C, Manfredi G, Springett R, Ten V, Galkin A (2018) The dependence of brain mitochondria reactive oxygen species production on oxygen level is linear, except when inhibited by antimycin A. J Neurochem 148:731-45. | Mouse | Nervous system | Ischemia-reperfusion Oxidative stress;RONS | |
| Jelenik 2018 Diabetes | 2018 | Jelenik T, Flögel U, Álvarez-Hernández E, Scheiber D, Zweck E, Ding Z, Rothe M, Mastrototaro L, Kohlhaas V, Kotzka J, Knebel B, Müller-Wieland D, Moellendorf S, Gödecke A, Kelm M, Westenfeld R, Roden M, Szendroedi J (2018) Insulin resistance and vulnerability to cardiac ischemia. Diabetes 67:2695-702. | Human Mouse | Heart | Ischemia-reperfusion | Cardiovascular Diabetes |
| Cooper 2018 Exp Physiol | 2018 | Cooper MA, McCoin C, Pei D, Thyfault JP, Koestler D, Wright DE (2018) Reduced mitochondrial reactive oxygen species production in peripheral nerves of mice fed a ketogenic diet. Exp Physiol 103:1206-12. | Mouse | Nervous system | Obesity Diabetes | |
| Pileggi 2018 Free Radic Biol Med | 2018 | Pileggi CA, Hedges CP, D'Souza RF, Durainayagam BR, Markworth JF, Hickey AJR, Mitchell CJ, Cameron-Smith D (2018) Exercise recovery increases skeletal muscle H2O2 emission and mitochondrial respiratory capacity following two-weeks of limb immobilization. Free Radic Biol Med 124:241-8. | Human | Skeletal muscle | Oxidative stress;RONS | |
| Kamunde 2018 Free Radic Biol Med | 2018 | Kamunde C, Sharaf M, MacDonald N (2018) H2O2 metabolism in liver and heart mitochondria: Low emitting-high scavenging and high emitting-low scavenging systems. Free Radic Biol Med 124:135-48. | Fishes | Heart Liver | Oxidative stress;RONS | |
| Falcao-Tebas 2018 J Physiol | 2018 | Falcão-Tebas F, Kuang J, Arceri C, Kerris JP, Andrikopoulos S, Marin EC, McConell GK (2018) Four weeks of exercise early in life reprograms adult skeletal muscle insulin resistance caused by paternal high fat diet. J Physiol 597:121-36. | Rat | Skeletal muscle | Obesity | |
| Robb 2018 J Biol Chem | 2018 | Robb EL, Hall AR, Prime TA, Eaton S, Szibor M, Viscomi C, James AM, Murphy MP (2018) Control of mitochondrial superoxide production by reverse electron transport at complex I. J Biol Chem 293:9869-79. https://doi.org/10.1074/jbc.RA118.003647 | Rat | Heart | Oxidative stress;RONS | |
| Smenes 2018 Interact Cardiovasc Thorac Surg | 2018 | Smenes BT, Bækkerud FH, Slagsvold KH, Hassel E, Wohlwend M, Pinho M, Høydal M, Wisløff U, Rognmo Ø, Wahba A (2018) Acute exercise is not cardioprotective and may induce apoptotic signalling in heart surgery: a randomized controlled trial. Interact Cardiovasc Thorac Surg 27:95-101. | Human | Heart | Ischemia-reperfusion | Aging;senescence Cardiovascular |
| Goncalves 2018 Neurotoxicology | 2018 | Gonçalves DF, Courtes AA, Hartmann DD, da Rosa PC, Oliveira DM, Soares FAA, Dalla Corte CL (2018) 6-Hydroxydopamine induces different mitochondrial bioenergetics response in brain regions of rat. Neurotoxicology 70:1-11. | Rat | Nervous system | Parkinson's Neurodegenerative | |
| Treberg 2018 Redox Biol | 2018 | Treberg JR, Braun K, Selseleh P (2018) Mitochondria can act as energy-sensing regulators of hydrogen peroxide availability. Redox Biol 20:483-88. | Rat | Skeletal muscle | ||
| Holland 2018 Cell Death Dis | 2018 | Holland OJ, Cuffe JSM, Dekker Nitert M, Callaway L, Kwan Cheung KA, Radenkovic F, Perkins AV (2018) Placental mitochondrial adaptations in preeclampsia associated with progression to term delivery. Cell Death Dis 9:1150. | Human | Genital | Ischemia-reperfusion | Other |
| Stepanova 2017 J Cereb Blood Flow Metab | 2017 | Stepanova A, Kahl A, Konrad C, Ten V, Starkov AS, Galkin A (2017) Reverse electron transfer results in a loss of flavin from mitochondrial complex I: Potential mechanism for brain ischemia-reperfusion injury. J Cereb Blood Flow Metab 37:3649-58. | Mouse | Nervous system | Ischemia-reperfusion | |
| Napa 2017 Int J Dent | 2017 | Napa K, Baeder AC, Witt JE, Rayburn ST, Miller MG, Dallon BW, Gibbs JL, Wilcox SH, Winden DR, Smith JH, Reynolds PR, Bikman BT (2017) LPS from P. gingivalis negatively alters gingival cell mitochondrial bioenergetics. Int J Dent 2017:2697210. | Human | Endothelial;epithelial;mesothelial cell | ||
| Sharaf 2017 Aquat Toxicol | 2017 | Sharaf MS, Stevens D, Kamunde C (2017) Zinc and calcium alter the relationship between mitochondrial respiration, ROS and membrane potential in rainbow trout (Oncorhynchus mykiss) liver mitochondria. Aquat Toxicol 189:170-83. | Fishes | Liver | ||
| De Velasco 2017 Br J Nutr | 2017 | de Velasco PC, Chicaybam G, Ramos-Filho DM, Dos Santos RMAR, Mairink C, Sardinha FLC, El-Bacha T, Galina A, Tavares-do-Carmo MDG (2017) Maternal intake of trans-unsaturated or interesterified fatty acids during pregnancy and lactation modifies mitochondrial bioenergetics in the liver of adult offspring in mice. Br J Nutr 118:41-52. | Mouse | Liver | ||
| Makrecka-Kuka 2017 Toxicol Lett | 2017 | Makrecka-Kuka M, Volska K, Antone U, Vilskersts R, Grinberga S, Bandere D, Liepinsh E, Dambrova M (2017) Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol Lett 267:32-8. | Mouse | Heart | ||
| Jang 2017 Biol Open | 2017 | Jang DH, Seeger SC, Grady ME, Shofer FC, Eckmann DM (2017) Mitochondrial dynamics and respiration within cells with increased open pore cytoskeletal meshes. Biol Open 6:1831-9. | Human | Endothelial;epithelial;mesothelial cell Fibroblast | Oxidative stress;RONS | Cancer |
| Lau 2017 Dissertation | 2017 | Lau G (2017) Adaptive variation of mitochondrial function in response to oxygen variability in intertidal sculpins (Cottidae, Actinopterygii). Dissertation p148. | Fishes | Nervous system | Hypoxia | |
| Sharaf 2017 Biochim Biophys Acta | 2017 | Sharaf MS, Stevens D, Kamunde C (2017) Mitochondrial transition ROS spike (mTRS) results from coordinated activities of complex I and nicotinamide nucleotide transhydrogenase. Biochim Biophys Acta 1858:955-65. | Mouse Fishes | Heart Liver | Oxidative stress;RONS | |
| Du 2017 Evolution | 2017 | Du SNN, Khajali F, Dawson NJ, Scott GR (2017) Hybridization increases mitochondrial production of reactive oxygen species in sunfish. Evolution 71:1643-52. | Fishes | Liver | Oxidative stress;RONS Ischemia-reperfusion | |
| Trewin 2017 PLOS ONE | 2017 | Trewin AJ, Levinger I, Parker L, Shaw CS, Serpiello FR, Anderson MJ, McConell GK, Hare DL, Stepto NK (2017) Acute exercise alters skeletal muscle mitochondrial respiration and H2O2 emission in response to hyperinsulinemic-euglycemic clamp in middle-aged obese men. PLOS ONE 12:e0188421. | Human | Skeletal muscle | Oxidative stress;RONS | Obesity |
| Cardoso 2017 Biochim Biophys Acta | 2017 | Cardoso GMF, Pletsch JT, Parmeggiani B, Grings M, Glanzel NM, Bobermin LD, Amaral AU, Wajner M, Leipnitz G (2017) Bioenergetics dysfunction, mitochondrial permeability transition pore opening and lipid peroxidation induced by hydrogen sulfide as relevant pathomechanisms underlying the neurological dysfunction characteristic of ethylmalonic encephalopathy. Biochim Biophys Acta 1863:2192-2201. | Rat | Nervous system | Permeability transition | Other |
| Ortega-Dominguez 2017 Food Chem Toxicol | 2017 | Ortega-Domínguez B, Aparicio-Trejo OE, García-Arroyo FE, León-Contreras JC, Tapia E, Molina-Jijón E, Hernández-Pando R, Sánchez-Lozada LG, Barrera-Oviedo D, Pedraza-Chaverri J (2017) Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem Toxicol 107:373-85. | ||||
| Briston 2017 Sci Rep | 2017 | Briston T, Roberts M, Lewis S, Powney B, M Staddon J, Szabadkai G, Duchen MR (2017) Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci Rep 7:10492. | Rat | Liver | Permeability transition | |
| Khalifa 2017 Physiol Rep | 2017 | Khalifa AR, Abdel-Rahman EA, Mahmoud AM, Ali MH, Noureldin M, Saber SH, Mohsen M, Ali SS (2017) Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep 5. pii: e13125. | Mouse | Heart Nervous system | Aging;senescence Cardiovascular Other | |
| Goedecke 2017 JMIR Res Protoc | 2017 | Goedecke JH, Mendham AE, Clamp L, Nono Nankam PA, Fortuin-de Smidt MC, Phiri L, Micklesfield LK, Keswell D, Woudberg NJ, Lecour S, Alhamud A, Kaba M, Lutomia FM, van Jaarsveld PJ, de Villiers A, Kahn SE, Chorell E, Hauksson J, Olsson T (2017) An exercise intervention to unravel the mechanisms underlying insulin resistance in a cohort of black South African women: Protocol for a randomized controlled trial. JMIR Res Protoc 03/10/2017:9098. | Human | Skeletal muscle Fat | Diabetes Obesity | |
| De Moura 2017 Neurotox Res | 2017 | de Moura Alvorcem L, da Rosa MS, Glänzel NM, Parmeggiani B, Grings M, Schmitz F, Wyse ATS, Wajner M, Leipnitz G (2017) Disruption of energy transfer and redox status by sulfite in hippocampus, striatum, and cerebellum of developing rats. Neurotox Res 32:264-75. | Rat | Nervous system | ||
| Lopez-Manzano 2017 Chem Res Toxicol | 2017 | Lopez-Manzano E, Cronican AA, Frawley KL, Peterson J, Pearce LL (2017) Cyanide scavenging by a cobalt Schiff-base macrocycle: a cost-effective alternative to corrinoids. Chem Res Toxicol 29:1011-9. | ||||
| Koning 2017 J Cereb Blood Flow Metab | 2017 | Koning G, Leverin AL, Nair S, Schwendimann L, Ek J, Carlsson Y, Gressens P, Thornton C, Wang X, Mallard C, Hagberg H (2017) Magnesium induces preconditioning of the neonatal brain via profound mitochondrial protection. J Cereb Blood Flow Metab 39:1038-55. | Rat | Ischemia-reperfusion Hypoxia | ||
| Newsom 2017 Am J Physiol Endocrinol Metab | 2017 | Newsom SA, Miller BF, Hamilton KL, Ehrlicher SE, Stierwalt HD, Robinson MM (2017) Long-term rates of mitochondrial protein synthesis are increased in mouse skeletal muscle with high fat feeding regardless of insulin sensitizing treatment. Am J Physiol Endocrinol Metab 313:552-62. | Mouse | Skeletal muscle | Diabetes Obesity | |
| Duicu 2017 Can J Physiol Pharmacol | 2017 | Duicu OM, Privistirescu A, Wolf A, Petruş A, Dănilă MD, Raţiu CD, Muntean DM, Sturza A (2017) Methylene blue improves mitochondrial respiration and decreases oxidative stress in a substrate-dependent manner in diabetic rat hearts. Can J Physiol Pharmacol 95:1376-82. | Rat | Heart | Diabetes Myopathy | |
| Xiong 2017 J Am Heart Assoc | 2017 | Xiong S, Wang B, Lin S, Zhang H, Li Y, Wei X, Cui Y, Wei X, Lu Z, Gao P, Li L, Zhao Z, Liu D, Zhu Z (2017) Activation of transient receptor potential melastatin subtype 8 attenuates cold-induced hypertension through ameliorating vascular mitochondrial dysfunction. J Am Heart Assoc 6. pii: e005495. | Mouse | Endothelial;epithelial;mesothelial cell | Temperature | |
| Timpani 2016 Neurotherapeutics | 2016 | Timpani CA, Trewin AJ, Stojanovska V, Robinson A, Goodman CA, Nurgali K, Betik AC, Stepto N, Hayes A, McConell GK, Rybalka E (2016) Attempting to compensate for reduced neuronal nitric oxide synthase protein with nitrate supplementation cannot overcome metabolic dysfunction but rather has detrimental effects in Dystrophin-deficient mdx muscle. Neurotherapeutics 14:429-46. | Mouse | Skeletal muscle | Other | |
| Bouitbir 2016 Antioxid Redox Signal | 2016 | Bouitbir J, Singh F, Charles AL, Schlagowski AI, Bonifacio A, Echaniz-Laguna A, Geny B, Krähenbühl S, Zoll J (2016) Statins trigger mitochondrial ROS-induced apoptosis in glycolytic skeletal muscle. Antioxid Redox Signal 24:84-98. | Rat | Skeletal muscle Other cell lines | Oxidative stress;RONS | Cardiovascular Myopathy |
| Gorbacheva 2016 International Symposium Mitochondrial Motility | 2016 | Gorbacheva OS, Strutynskyi RB, Khmil NV, Belosludtseva NV, Murzaeva SV, Korobeynikova MO, Alilova GA, Lezhnev EI, Mironova GD (2016) Study of the influence of flocalin on the energy and ion exchanges in rat liver mitochondria. International Symposium Mitochondrial Motility 73-7. | Rat | Liver | Cardiovascular | |
| Baeder 2016 Int J Dent | 2016 | Baeder AC, Napa K, Richardson ST, Taylor OJ, Andersen SG, Wilcox SH, Winden DR, Reynolds PR, Bikman BT (2016) Oral gingival cell cigarette smoke exposure induces muscle cell metabolic disruption. Int J Dent 2016:2763160. | Mouse | Skeletal muscle | ||
| Mezera 2016 Oxid Med Cell Longev | 2016 | Mezera V, Endlicher R, Kucera O, Sobotka O, Drahota Z, Cervinkova Z (2016) Effects of epigallocatechin gallate on tert-butyl hydroperoxide-induced mitochondrial dysfunction in rat liver mitochondria and hepatocytes. Oxid Med Cell Longev 7573131:8pp. | Rat | Liver | ||
| Alleman 2016 Am J Physiol Heart Circ Physiol | 2016 | Alleman RJ, Tsang AM, Ryan TE, Patteson DJ, McClung JM, Spangenburg EE, Shaikh SR, Neufer PD, Brown DA (2016) Exercise-induced protection against reperfusion arrhythmia involves stabilization of mitochondrial energetics. Am J Physiol Heart Circ Physiol 310:H1360-70. | Rat | Heart | Ischemia-reperfusion | Cardiovascular |
| Liepinsh 2016 Biochem J | 2016 | Liepinsh E, Makrecka-Kuka M, Volska K, Kuka J, Makarova E, Antone U, Sevostjanovs E, Vilskersts R, Strods A, Tars K, Dambrova M (2016) Long-chain acylcarnitines determine ischaemia/reperfusion-induced damage in heart mitochondria. Biochem J 473:1191-202. | Rat | Heart | Ischemia-reperfusion | |
| Abdel-Rahman 2016 Oxid Med Cell Longev | 2016 | Abdel-Rahman EA, Mokhtar A, Aaliya A, Radwan Y, Yasseen B, Al-Okda A, Atwa A, Elhanafy E, Habashy M, Ali SS (2016) Resolving contributions of oxygen-consuming and ROS-generating enzymes at the synapse. Oxid Med Cell Longev p19. | Mouse | Nervous system | Oxidative stress;RONS | |
| Du 2016 J Exp Biol | 2016 | Du SN, Mahalingam S, Borowiec BG, Scott GR (2016) Mitochondrial physiology and reactive oxygen species production are altered by hypoxia acclimation in killifish (Fundulus heteroclitus). J Exp Biol 219:1130-8. | Fishes | Liver | Oxidative stress;RONS | |
| Karlsson 2016 Shock | 2016 | Karlsson M, Hara N, Morata S, Sjövall F, Kilbaugh T, Hansson MJ, Uchino H, Elmér E (2016) Diverse and tissue-specific mitochondrial respiratory response in a mouse model of sepsis-induced multiple organ failure. Shock 45:404-10. | Mouse | Nervous system Liver | Oxidative stress;RONS | Sepsis |
| Power 2016 Am J Physiol Heart Circ Physiol | 2016 | Power AS, Pham T, Loiselle DS, Crossman DH, Ward ML, Hickey AJ (2016) Impaired ADP channeling to mitochondria and elevated reactive oxygen species in hypertensive hearts. https://doi.org/10.1152/ajpheart.00050.2016 | Rat | Heart | Cardiovascular | |
| Schiffer 2016 Am J Physiol Cell Physiol | 2016 | Schiffer TA, Peleli M, Sundqvist ML, Ekblom B, Lundberg JO, Weitzberg E, Larsen FJ (2016) Control of human energy expenditure by cytochrome c oxidase subunit IV-2. Am J Physiol Cell Physiol 311:C452-61. | Human | Skeletal muscle HEK | Oxidative stress;RONS Hypoxia | |
| Larsen 2016 FASEB J | 2016 | Larsen FJ, Schiffer TA, Ørtenblad N, Zinner C, Morales-Alamo D, Willis SJ, Calbet JA, Holmberg HC, Boushel R (2016) High-intensity sprint training inhibits mitochondrial respiration through aconitase inactivation. FASEB J 30:417-27. | Human | Skeletal muscle | Oxidative stress;RONS | |
| Makrecka-Kuka 2015 Biomolecules | 2015 | Makrecka-Kuka M, Krumschnabel G, Gnaiger E (2015) High-resolution respirometry for simultaneous measurement of oxygen and hydrogen peroxide fluxes in permeabilized cells, tissue homogenate and isolated mitochondria. https://doi.org/10.3390/biom5031319 | Human Mouse | Heart Nervous system HEK | Oxidative stress;RONS | |
| Kluckova 2015 Cell Death Dis | 2015 | Kluckova K, Sticha M, Cerny J, Mracek T, Dong L, Drahota Z, Gottlieb E, Neuzil J, Rohlena J (2015) Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation. Cell Death Dis 6:e1749. | Other mammals | Lung;gill Other cell lines Fibroblast | Cell death Oxidative stress;RONS | |
| Petrus 2015 Can J Physiol Pharmacol | 2015 | Petruş A, Duicu OM, Sturza A, Noveanu L, Kiss L, Dănilă M, Baczkó I, Muntean DM, Jost N (2015) Modulation of mitochondrial respiratory function and ROS production by novel benzopyran analogues. Can J Physiol Pharmacol 93:811-8. | Rat | Heart | Ischemia-reperfusion | |
| Krumschnabel 2015 Methods Mol Biol | 2015 | Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, Heidler J, Gauper K, Fasching M, Gnaiger E (2015) Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol Biol 1264:245-61. | Mouse | Nervous system | Oxidative stress;RONS | |
| Ferrera 2015 J Thorac Cardiovasc Surg | 2015 | Ferrera R, Benhabbouche S, Da Silva CC, Alam MR, Ovize M (2015) Delayed low pressure at reperfusion: A new approach for cardioprotection. J Thorac Cardiovasc Surg 150:1641-8. | Rat | Heart | Ischemia-reperfusion Permeability transition | |
| Beaudoin 2014 J Physiol | 2014 | Beaudoin MS, Perry CC, Arkell A, Chabowski A, Simpson JA, Wright DC, Holloway GP (2014) In the ZDF rat, impairments in mitochondrial palmitoyl-CoA respiratory kinetics that precede the development of diabetic cardiomyopathy are prevented by resveratrol supplementation. J Physiol 592:2519-33. | Rat | Heart | Diabetes | |
| Pham 2014 Am J Physiol | 2014 | Pham T, Loiselle D, Power A, Hickey AJ (2014) Mitochondrial inefficiencies and anoxic ATP hydrolysis capacities in diabetic rat heart. Am J Physiol 307:C499–507. | Rat | Heart | Ischemia-reperfusion Oxidative stress;RONS Mitochondrial disease | Diabetes Myopathy |
| Kukat 2014 PLoS Genet | 2014 | Kukat A, Dogan SA, Edgar D, Mourier A, Jacoby C, Maiti P, Mauer J, Becker C, Senft K, Wibom R, Kudin AP, Hultenby K, Flögel U, Rosenkranz S, Ricquier D, Kunz WS, Trifunovic A (2014) Loss of UCP2 attenuates mitochondrial dysfunction without altering ROS production and uncoupling activity. https://doi.org/10.1371/journal.pgen.1004385 | Mouse | Heart | Oxidative stress;RONS | |
| Hunter 2014 PhD Thesis | 2014 | Hunter FW (2014) Old target; new paradigm: Determinants of sensitivity to hypoxia-directed anticancer prodrugs. PhD Thesis:1-353. | Other mammals | CHO | Cancer | |
| Tretter 2014 Free Radic Biol Med | 2014 | Tretter L, Horvath G, Hölgyesi A, Essek F, Adam-Vizi V (2014) Enhanced hydrogen peroxide generation accompanies the beneficial bioenergetic effects of methylene blue in isolated brain mitochondria. Free Radic Biol Med 77:317-30. | Guinea pig | Nervous system | ||
| Iftikar 2014 Thesis University of Auckland | 2014 | Iftikar FI (2014) Testing the role of heart mitochondrial stability and function in heart failure of ectotherms exposed to heat stress. Thesis University of Auckland:167pp. | Fishes | Heart | Oxidative stress;RONS Temperature | |
| Soltysinska 2014 PLoS One | 2014 | Soltysinska E, Bentzen BH, Barthmes M, Hattel H, Thrush AB, Harper ME, Qvortrup K, Larsen FJ, Schiffer TA, Losa-Reyna J, Straubinger J, Kniess A, Thomsen MB, Brüggemann A, Fenske S, Biel M, Ruth P, Wahl-Schott C, Boushel RC, Olesen SP, Lukowski R (2014) KCNMA1 encoded cardiac BK channels afford protection against ischemia-reperfusion injury. PLoS One 9:e103402. | Mouse | Heart Skeletal muscle | Ischemia-reperfusion Oxidative stress;RONS | |
| Hara 2013 Eur J Anaesthesiol | 2013 | Hara N, Karlsson M, Sjövall F, Hansson Magnus J, Elmér E, Uchino H (2013) Early brain mitochondrial dysfunction in a mouse model of sepsis: 7AP4‐9. Eur J Anaesthesiol 30,112-112. | Mouse | Nervous system | Oxidative stress;RONS | Sepsis |
| Iftikar 2013 PLoS One | 2013 | Iftikar FI, Hickey AJ (2013) Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS One 8:e64120. | Fishes | Heart | Oxidative stress;RONS | |
| Jelenik 2013 Eur Heart J | 2013 | Jelenik T, Floegel U, Phielix E, Kaul K, Nowotny P, Partke HP, Schrader J, Roden M, Szendroedi S (2013) Non-alcoholic fatty liver disease and insulin resistance are associated with increased cardiac oxidative stress in mice. Eur Heart J 34:P5045. | Mouse | Heart | Oxidative stress;RONS | |
| Reilly 2013 J Exp Biol | 2013 | Reilly BD, Hickey AJ, Cramp RL, Franklin CE (2013) Decreased hydrogen peroxide production and mitochondrial respiration in skeletal muscle but not cardiac muscle of the green-striped burrowing frog, a natural model of muscle disuse. J Exp Biol 217:1087-93. | Amphibians | Heart Skeletal muscle | Oxidative stress;RONS | |
| Tretter 2012 Free Radic Biol Med | 2012 | Tretter Laszlo, Adam-Vizi Vera (2012) High Ca2+ load promotes hydrogen peroxide generation via activation of α-glycerophosphate dehydrogenase in brain mitochondria. Free Radic Biol Med 53:2119-30. | Guinea pig | Nervous system | Oxidative stress;RONS | |
| Hickey 2012 J Comp Physiol B | 2012 | Hickey AJ, Renshaw GM, Speers-Roesch B, Richards JG, Wang Y, Farrell AP, Brauner CJ (2012) A radical approach to beating hypoxia: depressed free radical release from heart fibers of the hypoxia-tolerant epaulette shark (Hemiscyllum ocellatum). J Comp Physiol B 182:91-100. | Fishes | Heart | Ischemia-reperfusion Oxidative stress;RONS | |
| Affourtit 2012 Methods Mol Biol | 2012 | Affourtit C, Quinlan CL, Brand MD (2012) Measurement of proton leak and electron leak in isolated mitochondria. Methods Mol Biol 810:165-82. | Rat | Skeletal muscle | Oxidative stress;RONS | |
| Hunter 2012 Biochem Pharmacol | 2012 | Hunter FW, Wang J, Patel R, Hsu HL, Hickey AJ, Hay MP, Wilson WR (2012) Homologous recombination repair-dependent cytotoxicity of the benzotriazine di-N-oxide CEN-209: Comparison with other hypoxia-activated prodrugs. Biochem Pharmacol 83:574–85. | CHO | Oxidative stress;RONS | ||
| Tretter 2007 J Neurochem | 2007 | Tretter L, Adam-Vizi V (2007) Uncoupling is without an effect on the production of reactive oxygen species by in situ synaptic mitochondria. J Neurochem 103:1864-71. | Other mammals | Nervous system | Oxidative stress;RONS |
Keywords: H2O2
- Bioblast links: Hydrogen peroxide - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Specific
- General