Garlid KD 2012 Abstract Bioblast
| Garlid KD (2012) Bioenergetics: A Physiological Overview. Mitochondr Physiol Network 17.12. |
Link: MiPNet17.12 Bioblast 2012 - Open Access
Garlid KD (2012)
Event: Bioblast 2012
The chemiosmotic theory, for which Peter Mitchell was awarded the Nobel Prize in Chemistry, was presented as a hypothesis far in advance of experimental evidence, and it stands as a glorious monument to the scientific method. Mitchell [1] proposed that nature uses protonic batteries to drive ATP synthesis and that biological energy conservation is essentially a problem in membrane transport. This was summarized in four postulates: 1) The electron transport system is vectorially oriented so that the energy of electron transport drives ejection of protons from the matrix, creating a proton electrochemical potential gradient. 2) The F1FO ATPase is also vectorially oriented so that the energy of ATP hydrolysis drives ejection of protons from the matrix. Because it is reversible, protons driven inward through the enzyme by the protonmotive force will cause ATP synthesis. 3) Ion leaks would short-circuit the protonmotive batteries, so the inner membrane must have a low diffusive permeability to ions in general and to protons in particular. 4) Cation leaks are compensated by electroneutral cation/proton antiporters, and low permeability for substrate anions is compensated by electroneutral anion exchange porters. Each of these postulates was, at the time, a radical departure from conventional wisdom. Postulates 3 and 4 form the basis for one aspect of mitochondrial physiology, but mitochondrial physiology is a rich and varied field, and includes cellular processes such as autophagy, fission/fusion, and apoptosis. In a brief talk, it will be necessary to focus on one aspect, and I will review recent progress in understanding the K+ cycle and its role in the cell.
• Keywords: Chemiosmosis, ATP synthesis and hydrolysis, K+ cycling
Labels:
Enzyme: Complex V; ATP Synthase"Complex V; ATP Synthase" is not in the list (Adenine nucleotide translocase, Complex I, Complex II;succinate dehydrogenase, Complex III, Complex IV;cytochrome c oxidase, Complex V;ATP synthase, Inner mt-membrane transporter, Marker enzyme, Supercomplex, TCA cycle and matrix dehydrogenases, ...) of allowed values for the "Enzyme" property. Regulation: Ion Homeostasis"Ion Homeostasis" is not in the list (Aerobic glycolysis, ADP, ATP, ATP production, AMP, Calcium, Coupling efficiency;uncoupling, Cyt c, Flux control, Inhibitor, ...) of allowed values for the "Respiration and regulation" property., ATP; ADP; AMP; PCr"ATP; ADP; AMP; PCr" is not in the list (Aerobic glycolysis, ADP, ATP, ATP production, AMP, Calcium, Coupling efficiency;uncoupling, Cyt c, Flux control, Inhibitor, ...) of allowed values for the "Respiration and regulation" property.
HRR: Theory
Affiliations and author contributions
Keith D Garlid: Dept of Biology, Portland State University, Portland, OR, USA; Email: [email protected]
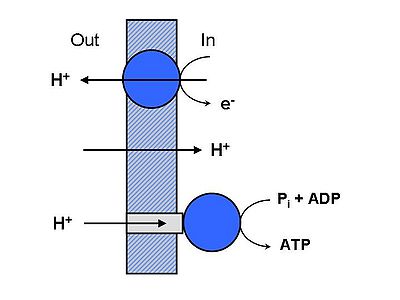
Figure 1
Coupling of electron transport with ATP synthesis. Protons are ejected electrogenically by the electron transport chain, generating a protonmotive force. This drives protons back through the ATPsynthase, leading to ATP production. Some of the energy is dissipated by electrophoretic back-diffusion, primarily of K+ and H+. Also necessary for ATP synthesis are the adenine nucleotide translocase, which catalyzes electrophoretic ATP/ADP exchange, and the phosphate transporter, which catalyzes electroneutral phosphate uptake. A considerable amount of mitochondrial physiology, including volume homeostasis, Ca2+ regulation, regulation of ROS production, and intramitochondrial signaling, is governed by inner membrane cation porters for Na+, K+ and Ca2+.