Difference between revisions of "Chemical potential"
| Line 18: | Line 18: | ||
''µ''<sub>H</sub><small>+</small> = -''RT''·ln(10)·pH | ''µ''<sub>H</sub><small>+</small> = -''RT''·ln(10)·pH | ||
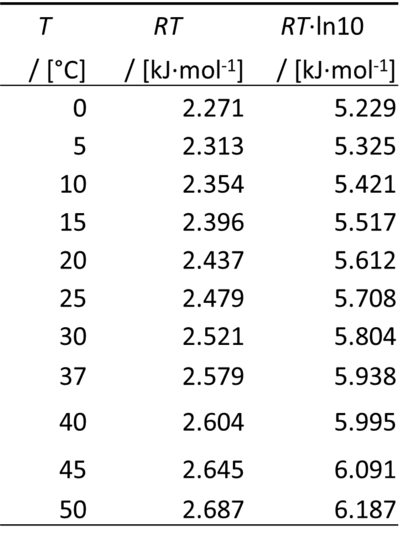
Therefore, for a difference of pH of -1 unit, Δ''µ''<sub>H</sub><small>+</small> equals | Therefore, for a difference of pH of -1 unit, Δ''µ''<sub>H</sub><small>+</small> equals ''RT''·ln(10): | ||
[[File:Table RT.png|left|400px|thumb|]] | [[File:Table RT.png|left|400px|thumb|]] | ||
0 °C = 273.15 K | 0 °C = 273.15 K | ||
ln(10) = 2.302585093 | ln(10) = 2.302585093 | ||
Revision as of 12:32, 18 October 2018
Description
The chemical potential of a substance B, µB [J/mol], is the partial derivative of Gibbs energy, G [J], per amount of B, nB [mol], at constant temperature, pressure, and composition other than that of B,
µB = (∂G/∂nB)T,p,nj≠B
The chemical potential of a solute in solution is the sum of the standard chemical potential measured under defined standard conditions and a concentration (activity)-dependent term,
µB = µB° + RT ln(aB)
The standard state for the solute is refered to ideal behaviour at standard concentration, c° = 1 mol/L, exhibiting infinitely diluted solution behaviour.
Abbreviation: µ
Reference: Cohen 2008 IUPAC Green Book
Communicated by Gnaiger E 2018-10-18
MitoPedia concepts: Ergodynamics
The proton chemical potential
- The standard chemical potential of protons is by defintion zero. Therefore, µH+ depends on the activity of protons only,
µH+ = RT ln(aH+)
Since pH = -lg(aH+), µH+ is related to pH as,
µH+ = -RT·ln(10)·pH
Therefore, for a difference of pH of -1 unit, ΔµH+ equals RT·ln(10):
0 °C = 273.15 K
ln(10) = 2.302585093