Arnold 2023 J Biol Chem: Difference between revisions
No edit summary |
No edit summary |
||
| (8 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Publication | {{Publication | ||
|title=Arnold PK, Finley LWS ( | |title=Arnold PK, Finley LWS (2023) Regulation and function of the mammalian tricarboxylic acid cycle. J Biol Chem 299:102838. https://doi.org/10.1016/j.jbc.2022.102838 | ||
|info=[https://pubmed.ncbi.nlm.nih.gov/36581208/ PMID: 36581208 Open Access] | |info=[https://pubmed.ncbi.nlm.nih.gov/36581208/ PMID: 36581208 Open Access] | ||
|authors=Arnold PK, Finley LWS | |authors=Arnold PK, Finley LWS | ||
|year= | |year=2023 | ||
|journal=J Biol Chem | |journal=J Biol Chem | ||
|abstract=The tricarboxylic acid (TCA) cycle, otherwise known as the Krebs cycle, is a central metabolic pathway that performs the essential function of oxidizing nutrients to support cellular bioenergetics. More recently, it has become evident that TCA cycle behavior is dynamic, and products of the TCA cycle can be co-opted in cancer and other pathologic states. In this review, we revisit the TCA cycle, including its potential origins and the history of its discovery. We provide a detailed accounting of the requirements for sustained TCA cycle function and the critical regulatory nodes that can stimulate or constrain TCA cycle activity. We also discuss recent advances in our understanding of the flexibility of TCA cycle wiring and the increasingly appreciated heterogeneity in TCA cycle activity exhibited by mammalian cells. Deeper insight into how the TCA cycle can be differentially regulated and, consequently, configured in different contexts will shed light on how this pathway is primed to meet the requirements of distinct mammalian cell states. | |abstract=The tricarboxylic acid (TCA) cycle, otherwise known as the Krebs cycle, is a central metabolic pathway that performs the essential function of oxidizing nutrients to support cellular bioenergetics. More recently, it has become evident that TCA cycle behavior is dynamic, and products of the TCA cycle can be co-opted in cancer and other pathologic states. In this review, we revisit the TCA cycle, including its potential origins and the history of its discovery. We provide a detailed accounting of the requirements for sustained TCA cycle function and the critical regulatory nodes that can stimulate or constrain TCA cycle activity. We also discuss recent advances in our understanding of the flexibility of TCA cycle wiring and the increasingly appreciated heterogeneity in TCA cycle activity exhibited by mammalian cells. Deeper insight into how the TCA cycle can be differentially regulated and, consequently, configured in different contexts will shed light on how this pathway is primed to meet the requirements of distinct mammalian cell states. | ||
|editor=Gnaiger E | |editor=Gnaiger E | ||
}} | }} | ||
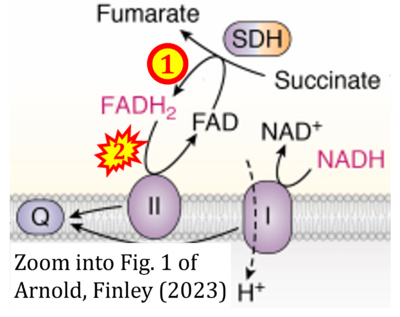
[[File:Arnold, Finley 2022 CORRECTION.png|right|400px]] | |||
{{Template:Correction FADH2 and S-pathway}} | |||
{{Labeling | |||
|enzymes=Complex II;succinate dehydrogenase | |||
}} | |||
: | |||
Latest revision as of 21:54, 28 August 2023
| Arnold PK, Finley LWS (2023) Regulation and function of the mammalian tricarboxylic acid cycle. J Biol Chem 299:102838. https://doi.org/10.1016/j.jbc.2022.102838 |
Arnold PK, Finley LWS (2023) J Biol Chem
Abstract: The tricarboxylic acid (TCA) cycle, otherwise known as the Krebs cycle, is a central metabolic pathway that performs the essential function of oxidizing nutrients to support cellular bioenergetics. More recently, it has become evident that TCA cycle behavior is dynamic, and products of the TCA cycle can be co-opted in cancer and other pathologic states. In this review, we revisit the TCA cycle, including its potential origins and the history of its discovery. We provide a detailed accounting of the requirements for sustained TCA cycle function and the critical regulatory nodes that can stimulate or constrain TCA cycle activity. We also discuss recent advances in our understanding of the flexibility of TCA cycle wiring and the increasingly appreciated heterogeneity in TCA cycle activity exhibited by mammalian cells. Deeper insight into how the TCA cycle can be differentially regulated and, consequently, configured in different contexts will shed light on how this pathway is primed to meet the requirements of distinct mammalian cell states.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase