Mosegaard 2020 Int J Mol Sci
| Mosegaard S, Dipace G, Bross P, Carlsen J, Gregersen N, Olsen RKJ (2020) Riboflavin deficiency-implications for general human health and inborn errors of metabolism. Int J Mol Sci 21:3847. https://doi.org/10.3390/ijms21113847 |
Mosegaard S, Dipace G, Bross P, Carlsen J, Gregersen N, Olsen RKJ (2020) Int J Mol Sci
Abstract: As an essential vitamin, the role of riboflavin in human diet and health is increasingly being highlighted. Insufficient dietary intake of riboflavin is often reported in nutritional surveys and population studies, even in non-developing countries with abundant sources of riboflavin-rich dietary products. A latent subclinical riboflavin deficiency can result in a significant clinical phenotype when combined with inborn genetic disturbances or environmental and physiological factors like infections, exercise, diet, aging and pregnancy. Riboflavin, and more importantly its derivatives, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), play a crucial role in essential cellular processes including mitochondrial energy metabolism, stress responses, vitamin and cofactor biogenesis, where they function as cofactors to ensure the catalytic activity and folding/stability of flavoenzymes. Numerous inborn errors of flavin metabolism and flavoenzyme function have been described, and supplementation with riboflavin has in many cases been shown to be lifesaving or to mitigate symptoms. This review discusses the environmental, physiological and genetic factors that affect cellular riboflavin status. We describe the crucial role of riboflavin for general human health, and the clear benefits of riboflavin treatment in patients with inborn errors of metabolism.
• Bioblast editor: Gnaiger E
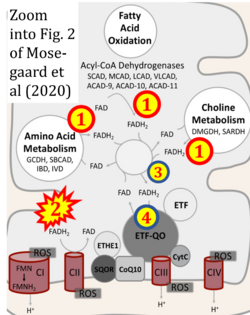
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase