Mathiyazakan 2023 Antimicrob Agents Chemother
| Mathiyazakan V, Wong CF, Harikishore A, Pethe K, Grüber G (2023) Cryo-electron microscopy structure of the Mycobacterium tuberculosis cytochrome bcc:aa3 supercomplex and a novel inhibitor targeting subunit cytochrome cI. Antimicrob Agents Chemother 67:e0153122. https://doi.org/10.1128/aac.01531-22 |
Mathiyazakan V, Wong CF, Harikishore A, Pethe K, Grueber G (2023) Antimicrob Agents Chemother
Abstract: The mycobacterial cytochrome bcc:aa3 complex deserves the name "supercomplex" since it combines three cytochrome oxidases-cytochrome bc, cytochrome c, and cytochrome aa3-into one supramolecular machine and performs electron transfer for the reduction of oxygen to water and proton transport to generate the proton motive force for ATP synthesis. Thus, the bcc:aa3 complex represents a valid drug target for Mycobacterium tuberculosis infections. The production and purification of an entire M. tuberculosis cytochrome bcc:aa3 are fundamental for biochemical and structural characterization of this supercomplex, paving the way for new inhibitor targets and molecules. Here, we produced and purified the entire and active M. tuberculosis cyt-bcc:aa3 oxidase, as demonstrated by the different heme spectra and an oxygen consumption assay. The resolved M. tuberculosis cyt-bcc:aa3 cryo-electron microscopy structure reveals a dimer with its functional domains involved in electron, proton, oxygen transfer, and oxygen reduction. The structure shows the two cytochrome cIcII head domains of the dimer, the counterpart of the soluble mitochondrial cytochrome c, in a so-called "closed state," in which electrons are translocated from the bcc to the aa3 domain. The structural and mechanistic insights provided the basis for a virtual screening campaign that identified a potent M. tuberculosis cyt-bcc:aa3 inhibitor, cytMycc1. cytMycc1 targets the mycobacterium-specific α3-helix of cytochrome cI and interferes with oxygen consumption by interrupting electron translocation via the cIcII head. The successful identification of a new cyt-bcc:aa3 inhibitor demonstrates the potential of a structure-mechanism-based approach for novel compound development.
• Bioblast editor: Gnaiger E
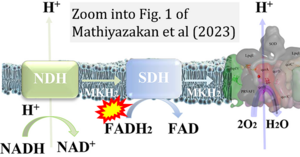
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase