Massart 2013 Curr Pathobiol Rep
| Massart J, Begriche K, Buron N, Porceddu M, Borgne-Sanchez A, Fromenty B (2013) Drug-induced inhibition of mitochondrial fatty acid oxidation and steatosis. Curr Pathobiol Rep 1:147–57. https://doi.org/10.1007/s40139-013-0022-y. |
Massart J, Begriche K, Buron N, Porceddu M, Borgne-Sanchez A, Fromenty B (2013) Curr Pathobiol Rep
Abstract: Drug-induced inhibition of mitochondrial fatty acid β-oxidation (mtFAO) is a key mechanism whereby drugs can induce steatosis. The type and severity of this liver lesion is dependent on the residual mtFAO flux. Indeed, a severe inhibition of mtFAO leads to microvesicular steatosis, hypoglycemia and liver failure, which can be favored by genetic predispositions. In contrast, moderate impairment of mtFAO can cause macrovacuolar steatosis, which is by itself a benign lesion. In the long-term, however, macrovacuolar steatosis can progress with some drugs to steatohepatitis. Interestingly, drugs that are more likely to cause steatohepatitis are those impairing the mitochondrial respiratory chain (MRC) activity. Indeed, MRC impairment favors not only hepatic fat accretion but also oxidative stress and lipid peroxidation. Drugs inhibiting mtFAO could be more toxic in obese patients with preexisting nonalcoholic fatty liver disease (NAFLD) since higher mtFAO is a key metabolic adaptation to curb fat accretion during NAFLD.
• Bioblast editor: Gnaiger E
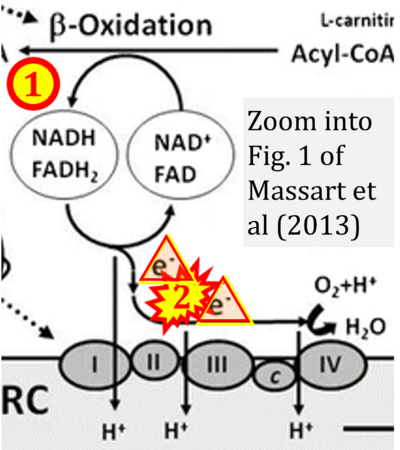
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase