Fogg 2011 Chin J Cancer
| Fogg VC, Lanning NJ, Mackeigan JP (2011) Mitochondria in cancer: at the crossroads of life and death. Chin J Cancer 30:526-39. https://doi.org/10.5732/cjc.011.10018 |
Fogg VC, Lanning NJ, Mackeigan JP (2011) Chin J Cancer
Abstract: Mitochondrial processes play an important role in tumor initiation and progression. In this review, we focus on three critical processes by which mitochondrial function may contribute to cancer: through alterations in glucose metabolism, the production of reactive oxygen species (ROS) and compromise of intrinsic apoptotic function. Alterations in cancer glucose metabolism include the Warburg effect, leading to a shift in metabolism away from aerobic respiration toward glycolysis, even when sufficient oxygen is present to support respiration. Such alterations in cellular metabolism may favor tumor cell growth by increasing the availability of biosynthetic intermediates needed for cellular growth and proliferation. Mutations in specific metabolic enzymes, namely succinate dehydrogenase, fumarate hydratase and the isocitrate dehydrogenases, have been linked to human cancer. Mitochondrial ROS may contribute to cancer via DNA damage and the activation of aberrant signaling pathways. ROS-dependent stabilization of the transcription factor hypoxia-inducible factor (HIF) may be a particularly important event for tumorigenesis. Compromised function of intrinsic apoptosis removes an important cellular safeguard against cancer and has been implicated in tumorigenesis, tumor metastasis, and chemoresistance. Each of the major mitochondrial processes is linked. In this review, we outline the connections between them and address ways these mitochondrial pathways may be targeted for cancer therapy.
• Bioblast editor: Gnaiger E
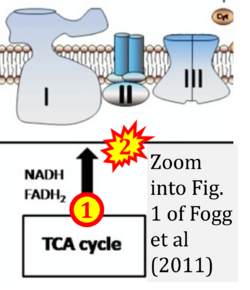
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: Pathology: Cancer
Enzyme: Complex II;succinate dehydrogenase