Egan 2023 Physiol Rev
| Egan B, Sharples AP (2023) Molecular responses to acute exercise and their relevance for adaptations in skeletal muscle to exercise training. Physiol Rev 103:2057-170. https://doi.org/10.1152/physrev.00054.2021 |
Egan B, Sharples AP (2023) Physiol Rev
Abstract: Repeated, episodic bouts of skeletal muscle contraction undertaken frequently as structured exercise training are a potent stimulus for physiological adaptation in many organs. Specifically, in skeletal muscle, remarkable plasticity is demonstrated by the remodeling of muscle structure and function in terms of muscular size, force, endurance, and contractile velocity as a result of the functional demands induced by various types of exercise training. This plasticity, and the mechanistic basis for adaptations to skeletal muscle in response to exercise training, are underpinned by activation and/or repression of molecular pathways and processes in response to each individual acute exercise session. These pathways include the transduction of signals arising from neuronal, mechanical, metabolic, and hormonal stimuli through complex signal transduction networks, which are linked to a myriad of effector proteins involved in the regulation of pre- and posttranscriptional processes, and protein translation and degradation processes. This review therefore describes acute exercise-induced signal transduction and the molecular responses to acute exercise in skeletal muscle including emerging concepts such as epigenetic pre- and posttranscriptional regulation and the regulation of protein translation and degradation. A critical appraisal of methodological approaches and the current state of knowledge informs a series of recommendations offered as future directions in the field.
• Bioblast editor: Gnaiger E
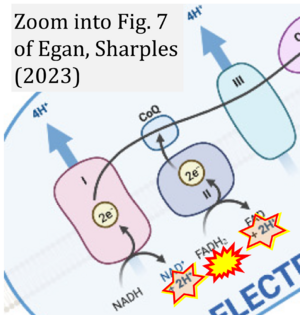
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels: MiParea: Exercise physiology;nutrition;life style
Enzyme: Complex II;succinate dehydrogenase