Chen 2014 Circ Res
| Chen YR, Zweier JL (2014) Cardiac mitochondria and reactive oxygen species generation. Circ Res 114:524-37. https://doi.org/10.1161/CIRCRESAHA.114.300559 |
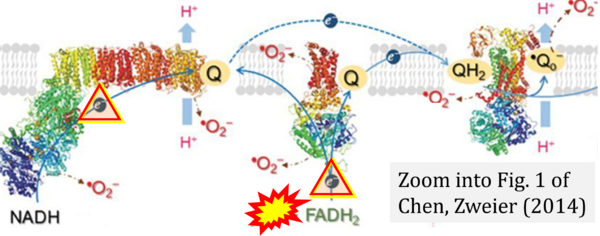
Chen YR, Zweier JL (2014) Circ Res
Abstract: Mitochondrial reactive oxygen species (ROS) have emerged as an important mechanism of disease and redox signaling in the cardiovascular system. Under basal or pathological conditions, electron leakage for ROS production is primarily mediated by the electron transport chain and the proton motive force consisting of a membrane potential (ΔΨ) and a proton gradient (ΔpH). Several factors controlling ROS production in the mitochondria include flavin mononucleotide and flavin mononucleotide-binding domain of complex I, ubisemiquinone and quinone-binding domain of complex I, flavin adenine nucleotide-binding moiety and quinone-binding pocket of complex II, and unstable semiquinone mediated by the Q cycle of complex III. In mitochondrial complex I, specific cysteinyl redox domains modulate ROS production from the flavin mononucleotide moiety and iron-sulfur clusters. In the cardiovascular system, mitochondrial ROS have been linked to mediating the physiological effects of metabolic dilation and preconditioning-like mitochondrial ATP-sensitive potassium channel activation. Furthermore, oxidative post-translational modification by glutathione in complex I and complex II has been shown to affect enzymatic catalysis, protein-protein interactions, and enzyme-mediated ROS production. Conditions associated with oxidative or nitrosative stress, such as myocardial ischemia and reperfusion, increase mitochondrial ROS production via oxidative injury of complexes I and II and superoxide anion radical-induced hydroxyl radical production by aconitase. Further insight into cellular mechanisms by which specific redox post-translational modifications regulate ROS production in the mitochondria will enrich our understanding of redox signal transduction and identify new therapeutic targets for cardiovascular diseases in which oxidative stress perturbs normal redox signaling.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase